Inhibitors of malaria parasite cyclic nucleotide phosphodiesterases block asexual blood-stage development and mosquito transmission
- PMID: 39642214
- PMCID: PMC11623267
- DOI: 10.1126/sciadv.adq1383
Inhibitors of malaria parasite cyclic nucleotide phosphodiesterases block asexual blood-stage development and mosquito transmission
Abstract
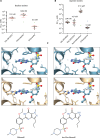
Cyclic nucleotide-dependent phosphodiesterases (PDEs) play essential roles in regulating the malaria parasite life cycle, suggesting that they may be promising antimalarial drug targets. PDE inhibitors are used safely to treat a range of noninfectious human disorders. Here, we report three subseries of fast-acting and potent Plasmodium falciparum PDEβ inhibitors that block asexual blood-stage parasite development and that are also active against human clinical isolates. Two of the inhibitor subseries also have potent transmission-blocking activity by targeting PDEs expressed during sexual parasite development. In vitro drug selection experiments generated parasites with moderately reduced susceptibility to the inhibitors. Whole-genome sequencing of these parasites detected no mutations in PDEβ but rather mutations in downstream effectors: either the catalytic or regulatory subunits of cyclic adenosine monophosphate-dependent protein kinase (PKA) or in the 3-phosphoinositide-dependent protein kinase that is required for PKA activation. Several properties of these P. falciparum PDE inhibitor series make them attractive for further progression through the antimalarial drug discovery pipeline.
Figures



References
-
- “World malaria report 2023” (Geneva License: CC BY-NC-SA 3.0 IGO., 2023).
-
- Baragana B., Hallyburton I., Lee M. C., Norcross N. R., Grimaldi R., Otto T. D., Proto W. R., Blagborough A. M., Meister S., Wirjanata G., Ruecker A., Upton L. M., Abraham T. S., Almeida M. J., Pradhan A., Porzelle A., Luksch T., Martinez M. S., Luksch T., Bolscher J. M., Woodland A., Norval S., Zuccotto F., Thomas J., Simeons F., Stojanovski L., Osuna-Cabello M., Brock P. M., Churcher T. S., Sala K. A., Zakutansky S. E., Jimenez-Diaz M. B., Sanz L. M., Riley J., Basak R., Campbell M., Avery V. M., Sauerwein R. W., Dechering K. J., Noviyanti R., Campo B., Frearson J. A., Angulo-Barturen I., Ferrer-Bazaga S., Gamo F. J., Wyatt P. G., Leroy D., Siegl P., Delves M. J., Kyle D. E., Wittlin S., Marfurt J., Price R. N., Sinden R. E., Winzeler E. A., Charman S. A., Bebrevska L., Gray D. W., Campbell S., Fairlamb A. H., Willis P. A., Rayner J. C., Fidock D. A., Read K. D., Gilbert I. H., A novel multiple-stage antimalarial agent that inhibits protein synthesis. Nature 522, 315–320 (2015). - PMC - PubMed
-
- McNamara C. W., Lee M. C., Lim C. S., Lim S. H., Roland J., Simon O., Yeung B. K., Chatterjee A. K., McCormack S. L., Manary M. J., Zeeman A. M., Dechering K. J., Kumar T. S., Henrich P. P., Gagaring K., Ibanez M., Kato N., Kuhen K. L., Fischli C., Nagle A., Rottmann M., Plouffe D. M., Bursulaya B., Meister S., Rameh L., Trappe J., Haasen D., Timmerman M., Sauerwein R. W., Suwanarusk R., Russell B., Renia L., Nosten F., Tully D. C., Kocken C. H., Glynne R. J., Bodenreider C., Fidock D. A., Diagana T. T., Winzeler E. A., Targeting Plasmodium PI(4)K to eliminate malaria. Nature 504, 248–253 (2013). - PMC - PubMed
-
- Rottmann M., McNamara C., Yeung B. K., Lee M. C., Zou B., Russell B., Seitz P., Plouffe D. M., Dharia N. V., Tan J., Cohen S. B., Spencer K. R., Gonzalez-Paez G. E., Lakshminarayana S. B., Goh A., Suwanarusk R., Jegla T., Schmitt E. K., Beck H. P., Brun R., Nosten F., Renia L., Dartois V., Keller T. H., Fidock D. A., Winzeler E. A., Diagana T. T., Spiroindolones, a potent compound class for the treatment of malaria. Science 329, 1175–1180 (2010). - PMC - PubMed
-
- Baker D. A., Stewart L. B., Large J. M., Bowyer P. W., Ansell K. H., Jimenez-Diaz M. B., El Bakkouri M., Birchall K., Dechering K. J., Bouloc N. S., Coombs P. J., Whalley D., Harding D. J., Smiljanic-Hurley E., Wheldon M. C., Walker E. M., Dessens J. T., Lafuente M. J., Sanz L. M., Gamo F. J., Ferrer S. B., Hui R., Bousema T., Angulo-Barturen I., Merritt A. T., Croft S. L., Gutteridge W. E., Kettleborough C. A., Osborne S. A., A potent series targeting the malarial cGMP-dependent protein kinase clears infection and blocks transmission. Nat. Commun. 8, 430 (2017). - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

