KRAS Variants Are Associated With Survival Outcomes and Genomic Alterations in Biliary Tract Cancers
- PMID: 39642327
- PMCID: PMC12305477
- DOI: 10.1200/PO.24.00263
KRAS Variants Are Associated With Survival Outcomes and Genomic Alterations in Biliary Tract Cancers
Abstract
Purpose: KRAS variants are associated with poor outcomes in biliary tract cancers (BTCs). This study assesses the prevalence of KRAS variants and their association with survival and recurrence in patients with intrahepatic cholangiocarcinoma (IHC), extrahepatic cholangiocarcinoma (EHC), and gallbladder adenocarcinoma (GB).
Methods: In this cross-sectional, single-institution study at Memorial Sloan Kettering, tumors from 985 patients treated between 2004 and 2022 with IHC, EHC, and GB who underwent either curative-intent resection or were treated with chemotherapy for unresectable disease were used for targeted sequencing.
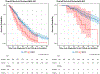
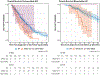
Results: Of the 985 patients sequenced, 15% had a KRAS mutation. Five hundred and seventy-two had unresectable disease (n = 395 IHC, n = 71 EHC, n = 106 GB) and 413 were treated with curative-intent resection (n = 175 IHC, n = 119 EHC, and n = 119 GB). Median follow-up time was 18 months (IQR, 11-31). KRAS G12D mutations were most common in IHC (38%) and EHC (37%) tumors. Mutations in SF3B1 co-occurred with mutant KRAS in IHC and EHC, with comutant resectable patients having worse survival after adjusting for tumor type (hazard ratio [HR], 4.04 [95% CI, 1.45 to 11.2]; P = .007). KRAS G12 mutations were associated with worse survival in patients with IHC compared with wild-type (WT) or other KRAS mutations, regardless of resection status (unresectable P < .001, resectable P = .011). After adjusting for clinical covariates, KRAS G12 mutations remained a prognostic indicator for patients with IHC compared with WT (HR, 1.99 [95% CI, 1.41 to 2.80]; P < .001).
Conclusion: The adverse impact of KRAS mutations in BTC is driven by G12 alterations in patients with IHC regardless of resection status, which was not observed in GB or EHC. There are unique comutational partners in distinct BTC subsets. These differences have important clinical implications in the era of KRAS-targeted therapeutics.
Figures









References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

