Tetrahydrobiopterin as a rheostat of cell resistance to oxidant injury
- PMID: 39642597
- PMCID: PMC11664145
- DOI: 10.1016/j.redox.2024.103447
Tetrahydrobiopterin as a rheostat of cell resistance to oxidant injury
Abstract
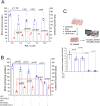
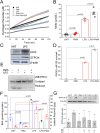
Tetrahydrobiopterin (BH4) deficiency is caused by genetic abnormalities that impair its biosynthesis and recycling, which trigger neurochemical, metabolic, and redox imbalances. Low BH4 levels are also associated with hypoxia, reperfusion reoxygenation, endothelial dysfunction, and other conditions that are not genetically determined. The exact cause of changes in BH4 in nongenetic disorders is not entirely understood, but a role for oxidant species has been implicated. The oxidation of BH4 generates several products, including 7,8-dihydrobiopterin (BH2), the accumulation of which is predicted in cells with low dihydrofolate reductase activity. The relative efficiency of oxidant species at causing variations in BH4/BH2 levels in cells furnished with several antioxidant enzymes has not yet been systematically analyzed. This study examined the quantitative changes of BH4/BH2 in cells challenged with several oxidants. We showed that BH2 is not a major product of treatments with hydrogen peroxide or RSL3, as indicated by the moderate effect of dihydrofolate reductase-inhibitor methotrexate on the accumulation of BH2. However, we found a net loss in BH4/BH2, suggesting that products other than BH2 were generated. These reactions were further examined in NOX4-expressing HEK cells producing hydrogen peroxide. These cells showed slightly decreased BH4/BH2 ratios compared with HEK wild-type cells, and, again, methotrexate treatment moderately increased BH2 levels. In contrast, peroxynitrite-producing RAW 264.7 cells showed dramatically decreased BH4 levels without BH2 accumulation. Following the activation of peroxynitrite production with PMA in lipopolysaccharide-treated cells, we also found a significant time-dependent decline in GTPCH-I protein levels. We conclude that hydrogen peroxide is the least effective oxidant species at decreasing intracellular BH4 levels, while peroxynitrite is highly effective by targeting GTPCH-I and BH4 directly. Moreover, we conclude that BH4/BH2 levels are not a determinant of RSL3 cytotoxicity.
Keywords: Antioxidants; BH2; Cytochrome c; Hydrogen peroxide; NOX4; Peroxynitrite; RSL3.
Copyright © 2024. Published by Elsevier B.V.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures





References
-
- Krzyaniak M.D., Eser B.E., Ellis H.R., Fitzpatrick P.F., McCracken J. Pulsed EPR study of amino acid and tetrahydropterin binding in a tyrosine hydroxylase nitric oxide complex: evidence for substrate rearrangements in the formation of the oxygen-reactive complex. Biochemistry. 2013;52(47):8430–8441. doi: 10.1021/bi4010914. - DOI - PMC - PubMed
-
- List B.M., Klösch B., Völker C., Gorren A.C., Sessa W.C., Werner E.R., Kukovetz W.R., Schmidt K., Mayer B. Characterization of bovine endothelial nitric oxide synthase as a homodimer with down-regulated uncoupled NADPH oxidase activity: tetrahydrobiopterin binding kinetics and role of haem in dimerization. Biochem. J. 1997;323(Pt 1):159–165. doi: 10.1042/bj3230159. Pt 1. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

