De-Mystifying the Clone-Censor-Weight Method for Causal Research Using Observational Data: A Primer for Cancer Researchers
- PMID: 39642890
- PMCID: PMC11623977
- DOI: 10.1002/cam4.70461
De-Mystifying the Clone-Censor-Weight Method for Causal Research Using Observational Data: A Primer for Cancer Researchers
Abstract
Background: Regulators and oncology healthcare providers are increasingly interested in using observational studies of real-world data (RWD) to complement clinical evidence from randomized controlled trials for informed decision-making. To generate valid evidence, RWD studies must be carefully designed to avoid systematic biases. The clone-censor-weight (CCW) method has been proposed to address immortal time and other time-related biases.
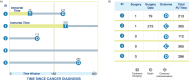
Methods: The objective of this manuscript is to de-mystify the CCW method for cancer researchers by describing and presenting its core components in an accessible and digestible format, using visualizations and examples from cancer-relevant studies. The CCW method has been applied in diverse settings, including investigations of the effects of surgery within a certain time after cancer diagnosis, the continuation of annual screening mammography, and chemotherapy duration on survival.
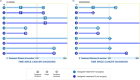
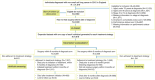
Results: The method handles complex data wherein the treatment group to which an individual belongs is unknown at the start of follow-up. The three steps of the CCW method involve cloning or duplicating the patient population and assigning one clone to each treatment strategy, artificially censoring the clones when their observed data are inconsistent with the assigned strategy and weighting the cloned and censored population to address selection bias created by the artificial censoring.
Conclusions: The CCW method is a powerful tool for designing RWD studies in cancer that are free from time-related biases and successfully, to the extent possible, emulate features of a randomized clinical trial.
Keywords: cancer screening; cancer treatment; causal inference; observational study; target trial emulation.
© 2024 The Author(s). Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
Dr. Gaber receives academic salary support from an educational fellowship from pharmaceutical company AbbVie Inc., but the company does not sponsor this study. Dr. Lund reports salary support from the UNC Center for Pharmacoepidemiology (current members: GlaxoSmithKline, UCB BioSciences, Takeda, AbbVie, Boehringer Ingelheim, Astellas) and from other pharmaceutical companies to UNC (Roche, Janssen) for unrelated research projects. Maribel Salas and Armen Ghazarian are current employees of Daichi Sankyo Inc., but the company does not sponsor this study. Xabier Garcia de Albeniz is a full‐time employee of RTI Health Solutions. Tatiane Ribeiro is a current employee at Takeda, but the company does not sponsor this study.
Figures

References
-
- Food and Drug Administration (FDA) , Framework for FDA's Real‐World Evidence Program (Silver Spring, MD: Food and Drug Administration, 2018), https://www.fda.gov/media/120060/download.
-
- European Medicines Agency (EMA) , Real‐World Evidence Framework to Support EU Regulatory Decision‐Making: Report on the Experience Gained With Regulator‐Led Studies (Amsterdam, The Netherlands: Heads of Medicine Agencies, 2021), https://www.ema.europa.eu/en/documents/report/real‐world‐evidence‐framew....
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

