Disease-modifying treatment and disability progression in subclasses of patients with primary progressive MS: results from the Big MS Data Network
- PMID: 39643429
- PMCID: PMC12171517
- DOI: 10.1136/jnnp-2024-334700
Disease-modifying treatment and disability progression in subclasses of patients with primary progressive MS: results from the Big MS Data Network
Abstract
Background: Effectiveness of disease-modifying treatment (DMT) in people affected by primary progressive multiple sclerosis (PPMS) is limited. Whether specific subgroups may benefit more from DMT in a real-world setting remains unclear. Our aim was to investigate the potential effect of DMT on disability worsening among patients with PPMS stratified by different disability trajectories.
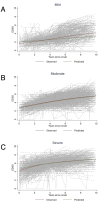
Methods: Within the framework of the Big MS Data network, we merged data from the Observatoire Français de la Sclérose en Plaques, the Swedish and Italian MS registries, and MSBase. We identified patients with PPMS that started DMT or were never treated during the observed period. Subpopulations with comparable baseline characteristics were selected by propensity score matching. Disability outcomes were analysed in time-to-recurrent event analyses, which were repeated in subclasses with different disability trajectories determined by latent class mixed models.
Results: Of the 3243 included patients, we matched 739 treated and 1330 untreated patients with a median follow-up of 3 years after pairwise censoring. No difference in the risk of confirmed disability worsening (CDW) was observed between the groups in the fully matched dataset (HR 1.11, 95% CI 0.97 to 1.23, p=0.127). However, we found a lower risk for CDW among the class of treated patients with an aggressive disability trajectory (n=360, HR 0.68, 95% CI 0.50 to 0.92, p=0.014).
Conclusions: In line with previous studies, our data suggest that DMT does not ameliorate disability worsening in PPMS, in general. However, we observed a beneficial effect of DMT on disability worsening in patients with aggressive predicted disability trajectories.
Keywords: MULTIPLE SCLEROSIS.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY. Published by BMJ Group.
Conflict of interest statement
Competing interests: JL’s institution has received research grants from Novartis, Biogen and Innosuisse–Swiss Innovation Agency, and honoraria for advisory boards and/or speaking fees from Novartis, Roche and Teva. He received conference travel support from Novartis and Bristol Myers Squibb. AS has nothing to disclose. SS has nothing to disclose. PB has nothing to disclose. SV has received non-personal consulting and lecturing fees, travel grants and unconditional research support from Biogen, Janssen, Merck, Novartis, Roche, Sandoz and Sanofi. MT has served on scientific advisory boards for Biogen, Novartis, Roche, Merck and BMS; has received speaker honoraria from Biogen, Roche, Sanofi, Merck, Alexion and Novartis; and has received research grants for her Institution from Biogen, Merck, Novartis and Roche. JH received honoraria for serving on advisory boards for Biogen, Bristol-Myers-Squibb, Janssen, Merck KgaA, Novartis, Sandoz and Sanofi-Genzyme and speaker’s fees from Biogen, Janssen, Novartis, Merck, Teva, Sandoz and Sanofi-Genzyme. He has served as PI for projects sponsored by, or received unrestricted research support from, Biogen, Bristol-Myers-Squibb, Janssen, Merck KgaA, Novartis, Roche and Sanofi-Genzyme. AG has nothing to disclose. RH has previously worked for Biogen, Ares Serono and Roche. LP has nothing to disclose (ORCID : 0000-0002-1506-3827). NK-H has nothing to dislose. PSS has nothing to disclose. TS received compensation from servicing on steering committees and advisory boards from Biogen. OGerlach has nothing to disclose. AP has nothing to disclose. MG has nothing to disclose. SEM has received speaker honoraria and consultant fees from Biogen Idec, Novartis, Merck, Janssen, Bristol-Meyers, Bayer, Sanofi Genzyme, Roche and Teva. PG has served in advisory boards for Novartis, EMD Serono, Roche, Biogen idec, Sanofi Genzyme, Pendopharm and has received grant support from Genzyme and Roche, has received research grants for his institution from Biogen idec, Sanofi Genzyme, EMD Serono. DH was supported by the Charles University: Cooperatio Program in Neuroscience, by the project National Institute for Neurological Research (Programme EXCELES, ID Project No. LX22NPO5107)—Funded by the European Union—Next Generation EU and by General University Hospital in Prague project MH CZ-DRO-VFN64165. She also received compensation for travel, speaker honoraria and consultant fees from Biogen Idec, Novartis, Merck, Bayer, Sanofi Genzyme, Roche and Teva, as well as support for research activities from Biogen Idec. CR-T has received consulting fees, speaker honoraria, support for attending meetings and/or travel, participation on advisory board and research grants for her institution from Biogen, Novartis, Sanofi, Bristol, Roche, Almirall, Janssen, Sandoz and Merck. IR has served on scientific advisory boards, received conference travel support and/or speaker honoraria from Roche, Novartis, Merck and Biogen. IR is supported by an MS Australia and the Trish Multiple Sclerosis Research Foundation. KB received speaker honoraria and/or education support from Biogen, Teva, Novartis, Genzyme-Sanofi, Roche, Merck and Alexion; has been a member of advisory boards for Merck and Biogen. JLS received travel compensation from Novartis, Biogen, Roche and Merck. Her institution receives the honoraria for talks and advisory board commitment as well as research grants from Biogen, Merck, Roche and Novartis. JLS-M accepted travel compensation from Novartis, Merck and Biogen, speaking honoraria from Biogen, Novartis, Sanofi, Merck, Almirall, Bayer and Teva and has participated in clinical trials by Biogen, Merck and Roche RA received honoraria as a speaker and for serving on scientific advisory boards from Bayer, Biogen, GSK, Merck, Novartis, Roche and Sanofi-Genzyme. JP accepted travel compensation from Novartis, Biogen, Genzyme, Teva and speaking honoraria from Biogen, Novartis, Genzyme and Teva. JK received speaker fees, research support, travel support and/or served on advisory boards by Swiss MS Society, Swiss National Research Foundation (320030_189140/1), University of Basel, Progressive MS Alliance, Alnylam, Bayer, Biogen, Bristol Myers Squibb, Celgene, Immunic, Merck, Neurogenesis, Novartis, Octave Bioscience, Quanterix, Roche, Sanofi, Stata DX. OGray received honoraria as consultant on scientific advisory boards for Genzyme, Biogen, Merck, Roche, and Novartis; has received travel grants from Biogen, Merck, Roche and Novartis; has participated in clinical trials by Biogen and Merck. Her institution has received research grant support from Biogen. GM has nothing to disclose. LM received honoraria for board and consulting from Roche, Teva, Biogen, Novartis, BMS, Merck and Sanofi. JC received fees for consulting, serving on a scientific advisory board, speaking, or other activities with Biogen, Novartis, Merck, Sanofi, Roche, Alexion and Horizon Therapeutics-Amgen JDS received consulting and lecturing fees, travel grants and unconditional research support from Biogen, Genzyme, Novartis, Roche, Sanofi Aventis and Teva Pharma. EM received consulting and lecturing fees from Alexion, Biogen, Horizon, Janssen, Merck Serono, Novartis, Roche, Sandoz, Sanofi-Genzyme, Teva Pharmaceuticals and research support from Biogen. AR received honoraria for meeting speaking from Merck, Alexion, Horizon Th and Sanofi Genzyme. AR received support for travelling from Biogen, Novartis and Merck. Her institution received research grants from Biogen, Roche, Sanofi-Genzyme and BMS. PL received consulting and lecturing fees, travel grants and unconditional research support from Biogen, Genzyme, Novartis, Merck Serono, Roche and Teva Pharma. HZ received consulting or lectures, and invitations for national and international congresses from Biogen, Merck, Teva, Sanofi-Genzyme, Novartis and Bayer, as well as research support from Teva and Roche and academic research grants from Académie de Médecine, LFSEP, FHU Imminent and ARSEP Foundation. AK has nothing to disclose. AvdW served on advisory boards and receives unrestricted research grants from Novartis, Biogen, Merck and Roche She has received speaker’s honoraria and travel support from Novartis, Roche and Merck. She receives grant support from the National Health and Medical Research Council of Australia and MS Research Australia. TK served on scientific advisory boards for MS International Federation and WHO, BMS, Roche, Janssen, Sanofi Genzyme, Novartis, Merck and Biogen, steering committee for Brain Atrophy Initiative by Sanofi Genzyme, received conference travel support and/or speaker honoraria from WebMD Global, Eisai, Novartis, Biogen, Roche, Sanofi-Genzyme, Teva, BioCSL and Merck and received research or educational event support from Biogen, Novartis, Genzyme, Roche, Celgene and Merck. HB received institutional (Monash University) funding from Biogen, Roche, Merck, Alexion and Novartis; has carried out contracted research for Novartis, Merck, Roche and Biogen; has taken part in speakers’ bureaus for Biogen, Novartis, Roche and Merck; has received personal compensation from Oxford Health Policy Forum for the Brain Health Steering Committee.
Figures

References
-
- Amezcua L. Progressive Multiple Sclerosis. Continuum (Mount Lawley) 2022;28:1083–103. doi: 10.1212/CON.0000000000001157. - DOI
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
