Conjugated fatty acids drive ferroptosis through chaperone-mediated autophagic degradation of GPX4 by targeting mitochondria
- PMID: 39643606
- PMCID: PMC11624192
- DOI: 10.1038/s41419-024-07237-w
Conjugated fatty acids drive ferroptosis through chaperone-mediated autophagic degradation of GPX4 by targeting mitochondria
Abstract
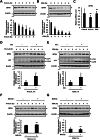
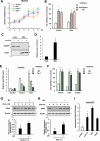
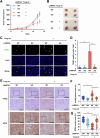
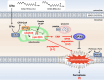
Conjugated fatty acids (CFAs) have been known for their anti-tumor activity. However, the mechanism of action remains unclear. Here, we identify CFAs as inducers of glutathione peroxidase 4 (GPX4) degradation through chaperone-mediated autophagy (CMA). CFAs, such as (10E,12Z)-octadecadienoic acid and α-eleostearic acid (ESA), induced GPX4 degradation, generation of mitochondrial reactive oxygen species (ROS) and lipid peroxides, and ultimately ferroptosis in cancer cell lines, including HT1080 and A549 cells, which were suppressed by either pharmacological blockade of CMA or genetic deletion of LAMP2A, a crucial molecule for CMA. Mitochondrial ROS were sufficient and necessary for CMA-dependent GPX4 degradation. Oral administration of an ESA-rich oil attenuated xenograft tumor growth of wild-type, but not that of LAMP2A-deficient HT1080 cells, accompanied by increased lipid peroxidation, GPX4 degradation and cell death. Our study establishes mitochondria as the key target of CFAs to trigger lipid peroxidation and GPX4 degradation, providing insight into ferroptosis-based cancer therapy.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests. Ethics approval and consent to participate: All animal experiments were approved by the ethics committee of Tohoku University (approval number: 2022PhA-008). All procedures were performed in accordance with the Guidelines for Animal Experiments of Tohoku University and the Japanese Government Animal Protection and Management Law. All authors checked the study and agreed to participate in the manuscript. Consent for publication: All authors checked the study and agreed.
Figures




References
MeSH terms
Substances
Grants and funding
- 21H00268/MEXT | Japan Society for the Promotion of Science (JSPS)
- JP21H02620/MEXT | Japan Society for the Promotion of Science (JSPS)
- 20K07011/MEXT | Japan Society for the Promotion of Science (JSPS)
- 20KK0361/MEXT | Japan Society for the Promotion of Science (JSPS)
- 23K06111/MEXT | Japan Society for the Promotion of Science (JSPS)
- 22J10429/MEXT | Japan Society for the Promotion of Science (JSPS)
- NA/Mitsubishi Foundation
- NA/Shimabara Science Promotion Foundation
- NA/Japan Foundation for Applied Enzymology
- NA/Takeda Science Foundation
- MH23KA3004/Ministry of Health, Labour and Welfare (Ministry of Health, Labour and Welfare, Japan)
LinkOut - more resources
Full Text Sources

