Coupling cellular drug-target engagement to downstream pharmacology with CeTEAM
- PMID: 39643609
- PMCID: PMC11624193
- DOI: 10.1038/s41467-024-54415-7
Coupling cellular drug-target engagement to downstream pharmacology with CeTEAM
Abstract
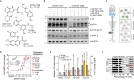
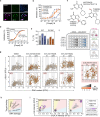
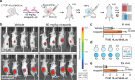
Cellular target engagement technologies enable quantification of intracellular drug binding; however, simultaneous assessment of drug-associated phenotypes has proven challenging. Here, we present cellular target engagement by accumulation of mutant as a platform that can concomitantly evaluate drug-target interactions and phenotypic responses using conditionally stabilized drug biosensors. We observe that drug-responsive proteotypes are prevalent among reported mutants of known drug targets. Compatible mutants appear to follow structural and biophysical logic that permits intra-protein and paralogous expansion of the biosensor pool. We then apply our method to uncouple target engagement from divergent cellular activities of MutT homolog 1 (MTH1) inhibitors, dissect Nudix hydrolase 15 (NUDT15)-associated thiopurine metabolism with the R139C pharmacogenetic variant, and profile the dynamics of poly(ADP-ribose) polymerase 1/2 (PARP1/2) binding and DNA trapping by PARP inhibitors (PARPi). Further, PARP1-derived biosensors facilitated high-throughput screening for PARP1 binders, as well as multimodal ex vivo analysis and non-invasive tracking of PARPi binding in live animals. This approach can facilitate holistic assessment of drug-target engagement by bridging drug binding events and their biological consequences.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare the following competing interests: N.C.K.V., B.D.G.P., and M.A. are inventors on a patent application describing CeTEAM and its uses (PCT/EP2019/073769). The remaining authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
- TJ2019-0036/Barncancerfonden (Swedish Childhood Cancer Foundation)
- PR2016-0101/Barncancerfonden (Swedish Childhood Cancer Foundation)
- 2019-01992/Stiftelsen Felix Mindus Bidrag till Leukemiforskningen
- 2020-01208/Loo och Hans Ostermans Stiftelse för Medicinsk Forskning (Loo and Hans Osterman Foundation for Medical Research)
- 2020-01685/Karolinska Institutet (Karolinska Institute)
- 2022-01749/Karolinska Institutet (Karolinska Institute)
- 2018-01655/Karolinska Institutet (Karolinska Institute)
- 21 0352 PT/Cancerfonden (Swedish Cancer Society)
- 201287/Cancerfonden (Swedish Cancer Society)
- CAN2018/0658/Cancerfonden (Swedish Cancer Society)
- NNF22OC0076798/Novo Nordisk Fonden (Novo Nordisk Foundation)
- NNF22OC0076798/Novo Nordisk Fonden (Novo Nordisk Foundation)
- CANCERPREV, 859860/EC | Horizon 2020 Framework Programme (EU Framework Programme for Research and Innovation H2020)
- CANCERPREV, 859860/EC | Horizon 2020 Framework Programme (EU Framework Programme for Research and Innovation H2020)
- BMA342854/Gouvernement du Canada | Canadian Institutes of Health Research (Instituts de Recherche en Santé du Canada)
- S16-0152/Svenska Sällskapet för Medicinsk Forskning (Swedish Society for Medical Research)
- 18292/Michael Smith Foundation for Health Research (MSFHR)
LinkOut - more resources
Full Text Sources
Miscellaneous

