Evoking stable and precise tactile sensations via multi-electrode intracortical microstimulation of the somatosensory cortex
- PMID: 39643730
- PMCID: PMC12176618
- DOI: 10.1038/s41551-024-01299-z
Evoking stable and precise tactile sensations via multi-electrode intracortical microstimulation of the somatosensory cortex
Abstract
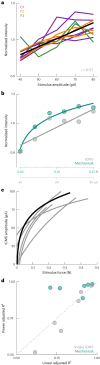
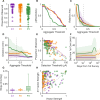
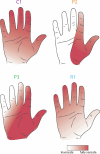
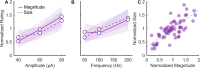
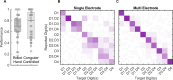
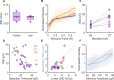
Tactile feedback from brain-controlled bionic hands can be partially restored via intracortical microstimulation (ICMS) of the primary somatosensory cortex. In ICMS, the location of percepts depends on the electrode's location and the percept intensity depends on the stimulation frequency and amplitude. Sensors on a bionic hand can thus be linked to somatotopically appropriate electrodes, and the contact force of each sensor can be used to determine the amplitude of a stimulus. Here we report a systematic investigation of the localization and intensity of ICMS-evoked percepts in three participants with cervical spinal cord injury. A retrospective analysis of projected fields showed that they were typically composed of a focal hotspot with diffuse borders, arrayed somatotopically in keeping with their underlying receptive fields and stable throughout the duration of the study. When testing the participants' ability to rapidly localize a single ICMS presentation, individual electrodes typically evoked only weak sensations, making object localization and discrimination difficult. However, overlapping projected fields from multiple electrodes produced more localizable and intense sensations and allowed for a more precise use of a bionic hand.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: N.G.H. and R.A.G. served as consultants for Blackrock Neurotech, Inc. at the time of the study. R.A.G. is also on the scientific advisory board of Neurowired LLC. M.L.B., J.L.C. and R.A.G. received research funding from Blackrock Neurotech, Inc. though that funding did not support the work presented here. A.R.S. serves as a consultant for Google DeepMind. The other authors declare no competing interests.
Figures










Update of
-
Biomimetic multi-channel microstimulation of somatosensory cortex conveys high resolution force feedback for bionic hands.bioRxiv [Preprint]. 2023 Jul 12:2023.02.18.528972. doi: 10.1101/2023.02.18.528972. bioRxiv. 2023. Update in: Nat Biomed Eng. 2025 Jun;9(6):935-951. doi: 10.1038/s41551-024-01299-z. PMID: 36824713 Free PMC article. Updated. Preprint.
References
-
- Johansson, R. S. & Flanagan, J. R. Coding and use of tactile signals from the fingertips in object manipulation tasks. Nat. Rev. Neurosci.10, 345–359 (2009). - PubMed
-
- Johansson, R. S. & Westling, G. Signals in tactile afferents from the fingers eliciting adaptive motor responses during precision grip. Exp. Brain Res.66, 141–154 (1987). - PubMed
-
- Monzée, J., Lamarre, Y. & Smith, A. M. The effects of digital anesthesia on force control using a precision grip. J. Neurophysiol.89, 672–683 (2003). - PubMed
-
- Wijk, U. & Carlsson, I. Forearm amputees’ views of prosthesis use and sensory feedback. J. Hand Ther.28, 269–278 (2015). - PubMed
-
- Flesher, S. N. et al. Intracortical microstimulation of human somatosensory cortex. Sci. Transl. Med.8, 361ra141 (2016). - PubMed
MeSH terms
Grants and funding
- SCI80308/United States Department of Defense | United States Army | Army Medical Command | Congressionally Directed Medical Research Programs (CDMRP)
- NS122333/U.S. Department of Health & Human Services | NIH | National Institute of Neurological Disorders and Stroke (NINDS)
- R35 NS122333/NS/NINDS NIH HHS/United States
- I01 RX002654/RX/RRD VA/United States
- NS107714/U.S. Department of Health & Human Services | NIH | National Institute of Neurological Disorders and Stroke (NINDS)
LinkOut - more resources
Full Text Sources

