Sequencing bispecific antibodies and CAR T cells for FL
- PMID: 39643999
- PMCID: PMC11665570
- DOI: 10.1182/hematology.2024000667
Sequencing bispecific antibodies and CAR T cells for FL
Abstract
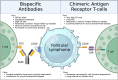
Treatment for relapsed/refractory (R/R) follicular lymphoma (FL) has evolved over recent years with the introduction of multiple novel immunotherapies: anti-CD3 × CD20 bispecific antibody (BsAb) T-cell engagers and anti-CD19 chimeric antigen receptor T cells (CAR T). Both drug classes are highly active, and their adverse event profiles overlap considerably, with cytokine release syndrome, cytopenias, and infections being most common. However, key differences include accessibility and logistical considerations as well as distinct neurologic toxicities, which make recommending a BsAb or CAR T a nuanced decision for each patient with R/R FL. Notably, patients could receive both classes of therapies in sequence; however, data guiding this decision are sparse. Considering the 3 most advanced agents in each class, we generally favor BsAbs before CAR T as the standard-of-care third-line treatment for the typical patient with R/R FL without concern for aggressive histologic transformation (HT). This is based on a 3-year follow-up of the mosunetuzumab phase 2 trial in R/R FL highlighting durable complete responses after a time-limited therapy with an acceptable safety profile for patients of all ages and reasonable performance status. We generally prioritize CAR T before BsAbs for patients with proven or suspected HT given the curative-potential of this approach based on trial data from R/R diffuse large B-cell lymphoma; it is unknown whether BsAbs offer the same long-term benefit in transformed FL. Overall, with the ability to personalize the sequencing of BsAbs and CAR T, the recently expanding portfolio of highly effective immunotherapies for R/R FL is poised to offer considerable benefit to this patient population.
Copyright © 2024 by The American Society of Hematology.
Conflict of interest statement
David A. Russler-Germain: research funding: Lymphoma Research Foundation, Institute for Follicular Lymphoma Innovation; consultancy: Regeneron; advisory board: AstraZeneca.
Nancy L. Bartlett: research funding: ADC Therapeutics, Bristol Myers Squibb, Celgene, Gilead/Kite Pharma, Merck, Millenium, Pharmacyclics, F. Hoffmann-La Roche/Genentech, Seattle Genetics; advisory committee: Foresight Diagnostics, Kite, F. Hoffmann-La Roche/Genentech, Seattle Genetics.
Figures


Similar articles
-
Comparative Analysis of Bispecific Antibodies and CAR T-Cell Therapy in Follicular Lymphoma.Eur J Haematol. 2025 Jan;114(1):4-16. doi: 10.1111/ejh.14335. Epub 2024 Oct 27. Eur J Haematol. 2025. PMID: 39462177 Free PMC article. Review.
-
Perspectives on T-cell engaging therapies in relapsed/refractory indolent non-Hodgkin lymphoma.Curr Opin Oncol. 2025 Sep 1;37(5):393-400. doi: 10.1097/CCO.0000000000001160. Epub 2025 Jun 5. Curr Opin Oncol. 2025. PMID: 40511598 Review.
-
[CAR T-cell therapy and bispecific antibodies for relapsed/refractory follicular lymphoma].Rinsho Ketsueki. 2025;66(6):424-431. doi: 10.11406/rinketsu.66.424. Rinsho Ketsueki. 2025. PMID: 40619225 Review. Japanese.
-
T-Cell Redirecting Strategies in Large B-Cell Lymphoma and Follicular Lymphoma.Hematol Oncol. 2025 Jun;43 Suppl 2:e70068. doi: 10.1002/hon.70068. Hematol Oncol. 2025. PMID: 40517438 Review.
-
Bispecific Antibodies for Non-Hodgkin Lymphoma Treatment.Curr Treat Options Oncol. 2022 Feb;23(2):155-170. doi: 10.1007/s11864-021-00925-1. Epub 2022 Feb 19. Curr Treat Options Oncol. 2022. PMID: 35182296 Review.
Cited by
-
Two Are Better than One: The Bi-Specific Antibody Mosunetuzumab Reveals an Improved Immune Response of Vγ9Vδ2 T Cells Targeting CD20 in Malignant B Cells in Comparison to the Mono-Specific Antibody Obinutuzumab.Int J Mol Sci. 2025 Jan 31;26(3):1262. doi: 10.3390/ijms26031262. Int J Mol Sci. 2025. PMID: 39941030 Free PMC article.
References
-
- Casulo C, Herold M, Hiddemann W, et al.. Risk factors for and outcomes of follicular lymphoma histological transformation at first progression in the GALLIUM study. Clin Lymphoma Myeloma Leuk. 2023;23(1):40-48. - PubMed
-
- Fowler NH, Dickinson M, Dreyling M, et al.. Tisagenlecleucel in adult relapsed or refractory follicular lymphoma: the phase 2 ELARA trial. Nat Med. 2022;28(2):325-332. - PubMed
-
- Jacobson CA, Chavez JC, Sehgal AR, et al.. Axicabtagene ciloleucel in relapsed or refractory indolent non-Hodgkin lymphoma (ZUMA-5): a single- arm, multicentre, phase 2 trial. Lancet Oncol. 2022;23(1):91-103. - PubMed
-
- Budde LE, Sehn LH, Matasar M, et al.. Safety and efficacy of mosunetuzumab, a bispecific antibody, in patients with relapsed or refractory follicular lymphoma: a single-arm, multicentre, phase 2 study. Lancet Oncol. 2022;23(8):1055-1065. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

