Biological, as opposed to classic antipsoriatic drug or apremilast, treatment mitigates the risk of death and cardiovascular disease in psoriasis
- PMID: 39644770
- PMCID: PMC11665659
- DOI: 10.1016/j.ebiom.2024.105485
Biological, as opposed to classic antipsoriatic drug or apremilast, treatment mitigates the risk of death and cardiovascular disease in psoriasis
Abstract
Background: Cardiovascular comorbidity increases morbidity and mortality in psoriasis. Systemic treatments, particularly biologics, are effective in alleviating skin and joint inflammation. Conversely, the impact of systemic therapy on cardiovascular disease risk and mortality in psoriasis remains uncertain.
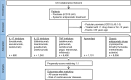
Methods: Impact of systemic treatments on all-cause mortality and cardiovascular disease risk in psoriasis patients' electronic health records (EHRs) from TriNetX was assessed. Treatment categories included apremilast, IL-17 inhibitors (IL-17i), IL23i, TNFi, and classic antipsoriatic drugs. Index event was the first prescription of each treatment, requiring a two-year continuous treatment with exclusion of other systemic antipsoriatic drugs. Propensity-score matching was used to improve comparability. Sensitivity analyses ensured study robustness.
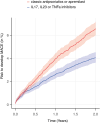
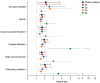
Findings: In descriptive analysis, all-cause mortality rates were 0.61% (classic antipsoriatics, n = 7929), 0.91% (apremilast, n = 1101), 0.00% (IL17i, n = 677), 0.81% (IL23i, n = 1242), and 0.20% (TNFi, n = 6468). Major adverse cardiac events (MACE) were documented in 8.49% (classic antipsoriatics), 5.14% (apremilast), 2.99% (IL17i), 2.09% (IL23i), and 3.74% (TNFi) EHRs. Propensity-score matching showed all-cause mortality rates of 0.23% for any biologic vs. 0.49% for classic antipsoriatics or apremilast, resulting in an HR of 2.21 (95% CI 1.21-3.71, p = 0.0073). MACE risk was also higher with classic antipsoriatics or apremilast (HR 1.66, CI 1.43-1.93, p < 0.0001). The majority of findings were consistent across all four sensitivity analyses. No significant differences in all-cause mortality or MACE risk were observed among biologics.
Interpretation: Biological treatment, as opposed to classic antipsoriatic drugs or apremilast, reduces risk of death and cardiovascular disease in psoriasis. Prospective trials are required to validate these findings.
Funding: DFG: EXC 2167 and LU 877/25-1. State of Schleswig Holstein: Excellence-Chair Program.
Keywords: Cardiovascular; Comorbidity; Major adverse cardiac events (MACE); Mortality; Psoriasis; TriNetX.
Copyright © 2024 The Author(s). Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of interests Diamant Thaci has received honoraria or fees for serving on advisory boards, as a speaker, as a consultant from AbbVie, Amgen, Almirall, Beiersdorf, Bristol-Meiers-Squibb, Boehringer Ingelheim, Galapagos, Leo Pharma, Merck Sharp & Dohme, Morphosys, Lilly, Novartis, Janssen-Cilag, Pfizer, Regeneron, Sanofi, Hexal, Sun Pharmaceuticals, and UCB and grants from Leo Pharma and Novartis. Gema Hernandez is an employee of TriNetX. Henner Zirpel has received support for attending meetings and/or travel from Pfizer, UCB Pharma, Almirall, Janssen, TriNetX. Ralf J. Ludwig has received honoraria for speaking or consulting or has obtained research grants from Monasterium Laboratories, Novartis, Lilly, Bayer, Dompe, Synthon, Argen-X, TriNetX, and Incyte during the last 3 years. All other authors declare no conflict of interest.
Figures
References
-
- Global report on psoriasis. 2016.
-
- McDonald C.J., Calabresi P. Occlusive vascular disease in psoriatic patients. N Engl J Med. 1973;288:912. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

