Reactive oxygen species: Janus-faced molecules in the era of modern cancer therapy
- PMID: 39645234
- PMCID: PMC11629020
- DOI: 10.1136/jitc-2024-009409
Reactive oxygen species: Janus-faced molecules in the era of modern cancer therapy
Abstract
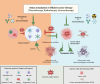
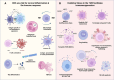
Oxidative stress, that is, an unbalanced increase in reactive oxygen species (ROS), contributes to tumor-induced immune suppression and limits the efficacy of immunotherapy. Cancer cells have inherently increased ROS production, intracellularly through metabolic perturbations and extracellularly through activation of NADPH oxidases, which promotes cancer progression. Further increased ROS production or impaired antioxidant systems, induced, for example, by chemotherapy or radiotherapy, can preferentially kill cancer cells over healthy cells. Inflammatory cell-derived ROS mediate immunosuppressive effects of myeloid-derived suppressor cells and activated granulocytes, hampering antitumor effector cells such as T cells and natural killer (NK) cells. Cancer therapies modulating ROS levels in tumors may thus have entirely different consequences when targeting cancer cells versus immune cells. Here we discuss the possibility of developing more efficient cancer therapies based on reduction-oxidation modulation, as either monotherapies or in combination with immunotherapy. Short-term, systemic administration of antioxidants or drugs blocking ROS production can boost the immune system and act in synergy with immunotherapy. However, prolonged use of antioxidants can instead enhance tumor progression. Alternatives to systemic antioxidant administration are under development where gene-modified or activated T cells and NK cells are shielded ex vivo against the harmful effects of ROS before the infusion to patients with cancer.
Keywords: Immune modulatory; Immunosuppression; Immunotherapy; Tumor microenvironment - TME.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: The following information is relevant for the content of this review; SLW, ESJA and RK are inventors on a patent involving redox modulation for immunotherapy, which is further being commercialized by SLW and RK. ESJA also has patents on TXNRD1 inhibitors for use in cancer chemotherapeutics.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
