Macrophage metabolic reprogramming ameliorates diabetes-induced microvascular dysfunction
- PMID: 39647239
- PMCID: PMC11667058
- DOI: 10.1016/j.redox.2024.103449
Macrophage metabolic reprogramming ameliorates diabetes-induced microvascular dysfunction
Abstract
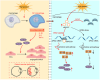
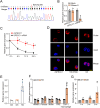
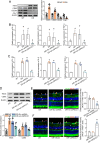
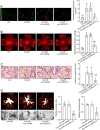
Macrophages play an important role in the development of vascular diseases, with their homeostasis closely linked to metabolic reprogramming. This study aims to explore the role of circular RNA-mediated epigenetic remodeling in maintaining macrophage homeostasis during diabetes-induced microvascular dysfunction. We identified a circular RNA, circRNA-sperm antigen with calponin homology and coiled-coil domains 1 (cSPECC1), which is significantly up-regulated in diabetic retinas and in macrophages under diabetic stress. cSPECC1 knockdown in macrophages attenuates M1 macrophage polarization and disrupts macrophage-endothelial crosstalk in vitro. cSPECC1 knockdown in macrophages mitigates diabetes-induced retinal inflammation and ameliorates retinal vascular dysfunction. Mechanistically, cSPECC1 regulates GPX2 expression by recruiting eIF4A3, enhancing GPX2 mRNA stability and altering arachidonic acid metabolism. The metabolic intermediate 12-HETE has emerged as a key mediator, regulating both macrophage homeostasis and the crosstalk between macrophages and endothelial cells. Exogenous 12-HETE supplementation interrupts the anti-angiogenic effects of cSPECC1 knockdown. Collectively, circSPECC1 emerges as a novel regulator of macrophage-mediated vascular integrity and inflammation. Targeting the metabolic reprogramming of macrophages presents a promising therapeutic strategy for mitigating diabetes-induced vascular dysfunction.
Keywords: Circular RNAs; Diabetic retinopathy; Macrophage homeostasis; Metabolic reprogramming; Vascular dysfunction.
Copyright © 2024 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




References
-
- Li X., Sun X., Carmeliet P. Hallmarks of endothelial cell metabolism in health and disease. Cell Metabol. 2019;30(3):414–433. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

