Zanubrutinib Versus Bendamustine and Rituximab in Patients With Treatment-Naïve Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma: Median 5-Year Follow-Up of SEQUOIA
- PMID: 39647999
- PMCID: PMC11855994
- DOI: 10.1200/JCO-24-02265
Zanubrutinib Versus Bendamustine and Rituximab in Patients With Treatment-Naïve Chronic Lymphocytic Leukemia/Small Lymphocytic Lymphoma: Median 5-Year Follow-Up of SEQUOIA
Abstract
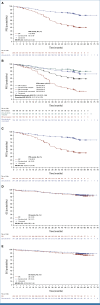
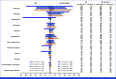
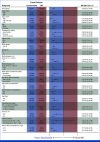
Clinical trials frequently include multiple end points that mature at different times. The initial report, typically based on the primary end point, may be published when key planned co-primary or secondary analyses are not yet available. Clinical Trial Updates provide an opportunity to disseminate additional results from studies, published in JCO or elsewhere, for which the primary end point has already been reported.SEQUOIA (ClinicalTrials.gov identifier: NCT03336333) is a phase III, randomized, open-label trial that compared the oral Bruton tyrosine kinase inhibitor zanubrutinib to bendamustine plus rituximab (BR) in treatment-naïve patients with chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL). The initial prespecified analysis (median follow-up, 26.2 months) and subsequent analysis (43.7 months) found superior progression-free survival (PFS; the primary end point) in patients who received zanubrutinib compared with BR. At a median follow-up of 61.2 months, median PFS was not reached in zanubrutinib-treated patients; median PFS was 44.1 months in BR-treated patients (hazard ratio [HR], 0.29; one-sided P = .0001). Prolonged PFS was seen with zanubrutinib versus BR in patients with mutated immunoglobulin heavy-chain variable region (IGHV) genes (HR, 0.40; one-sided P = .0003) and unmutated IGHV genes (HR, 0.21 [95% CI, 0.14 to 0.33]; one-sided P < .0001). Median overall survival (OS) was not reached in either treatment arm; estimated 60-month OS rates were 85.8% and 85.0% in zanubrutinib- and BR-treated patients, respectively. No new safety signals were detected. Adverse events were as expected with zanubrutinib; rate of atrial fibrillation was 7.1%. At a median follow-up of 61.2 months, the results supported the initial SEQUOIA findings and suggested that zanubrutinib was a favorable treatment option for untreated patients with CLL/SLL.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
No other potential conflicts of interest were reported.
Figures
References
-
- Shadman M: Diagnosis and treatment of chronic lymphocytic leukemia: A review. JAMA 329:918-932, 2023 - PubMed
-
- Guo Y, Liu Y, Hu N, et al. : Discovery of zanubrutinib (BGB-3111), a novel, potent, and selective covalent inhibitor of Bruton's tyrosine kinase. J Med Chem 62:7923-7940, 2019 - PubMed
-
- Brukinsa (Zanubrutinib) [package insert]. San Mateo, CA, BeiGene USA, 2023
-
- Brukinsa (zanubrutinib) : [summary of product characteristics]. Dublin, Ireland, BeiGene Ireland Limited, 2024
-
- Tam CS, Brown JR, Kahl BS, et al. : Zanubrutinib versus bendamustine and rituximab in untreated chronic lymphocytic leukaemia and small lymphocytic lymphoma (SEQUOIA): A randomised, controlled, phase 3 trial. Lancet Oncol 23:1031-1043, 2022 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

