Outcome of Patients With Lupus Nephritis Treated With an Anti-CD40 Monoclonal Antibody According to Kidney Biopsy Features
- PMID: 39648337
- PMCID: PMC12123251
- DOI: 10.1002/art.43076
Outcome of Patients With Lupus Nephritis Treated With an Anti-CD40 Monoclonal Antibody According to Kidney Biopsy Features
Abstract
Objective: A phase 2 trial tested different doses of the anti-CD40 monoclonal antibody BI 655064 as an add-on therapy to the standard of care in patients with class III or IV lupus nephritis (LN) with active disease. A post hoc analysis showed a potential benefit of the higher tested doses (180 and 240 mg) versus a low dose (120 mg) or placebo. We investigated whether the treatment effect of BI 655064 on kidney outcomes may be modified by the presence of glomerular monocytes, a target for this drug with a well-known role in LN pathogenesis.
Methods: One hundred one renal biopsies of patients with LN enrolled in the BI 655064 trial were scored centrally. The estimated glomerular filtration rate (eGFR), spot urine protein/urine creatinine ratio (UP/UC), and complete renal response (CRR) were evaluated over 52 weeks. Patients were divided according to a "better" or "worse" performance than the average of all patients in the cohort, predicted by a mixed model for repeated measurements. Logistic regression models adjusted for potential confounders were used to assess the association between different treatment doses and outcomes according to the presence or absence of monocytes.
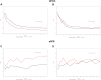
Results: A higher BI 655064 dose (180 or 240 mg) was associated with better outcomes of UP/UC and CRR when glomerular monocytes were present in kidney biopsy samples (odds ratio [OR] 3.66 [95% confidence interval (CI) 1.09-12.3], P = 0.04; OR 4.58 [95% CI 1.24-16.9], P = 0.02). A trend toward improved eGFR was also observed in these patients (at 52 weeks, P = 0.08).
Conclusion: In LN kidney biopsy samples with glomerular monocytes, high-dose BI 655064 treatment improved proteinuria at 52 weeks and resulted in a higher CRR compared to biopsy samples without glomerular monocytes. Histologic features may guide the choice of treatment for individual patients with LN.
© 2024 The Author(s). Arthritis & Rheumatology published by Wiley Periodicals LLC on behalf of American College of Rheumatology.
Figures
References
-
- Mok CC, Tang SSK. Incidence and predictors of renal disease in Chinese patients with systemic lupus erythematosus. Am J Med 2004;117(10):791–795. - PubMed
-
- Mok CC. Towards new avenues in the management of lupus glomerulonephritis. Nat Rev Rheumatol 2016;12(4):221–234. - PubMed
-
- Ramanujam M, Steffgen J, Visvanathan S, et al. Phoenix from the flames: rediscovering the role of the CD40–CD40L pathway in systemic lupus erythematosus and lupus nephritis. Autoimmun Rev 2020;19(11):102668. - PubMed
-
- Bajema IM, Wilhelmus S, Alpers CE, et al. Revision of the International Society of Nephrology/Renal Pathology Society classification for lupus nephritis: clarification of definitions, and modified National Institutes of Health activity and chronicity indices. Kidney Int 2018;93(4):789–796. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

