Safety and immunogenicity of CD40.HIVRI.Env, a dendritic cell-based HIV vaccine, in healthy HIV-uninfected adults: a first-in-human randomized, placebo-controlled, dose-escalation study (ANRS VRI06)
- PMID: 39649135
- PMCID: PMC11625018
- DOI: 10.1016/j.eclinm.2024.102845
Safety and immunogenicity of CD40.HIVRI.Env, a dendritic cell-based HIV vaccine, in healthy HIV-uninfected adults: a first-in-human randomized, placebo-controlled, dose-escalation study (ANRS VRI06)
Abstract
Background: Current HIV prophylactic vaccines evaluate HIV Env as purified proteins. CD40.HIVRI.Env is an innovative antigen delivery targeting gp140 Env from HIV Clade C 96ZM651 to CD40-expressing antigen-presenting cells, thus harnessing the intrinsic immune-stimulant properties. DNA-HIV-PT123 vaccine encodes 96ZM651 gp140/Gag and 97CN54 Pol/Nef.
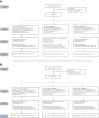
Methods: Seventy-two HIV-negative volunteers were enrolled between 05/2021 and 10/2022 in a phase 1 placebo-controlled trial conducted in France and Switzerland (N° EudraCT: 2020-001814-40; NCT04842682). Volunteers were randomized (5:1 active versus placebo) in groups receiving either 0.3, 1.0, or 3.0 mg CD40.HIVRI.Env (Hiltonol® adjuvanted) alone or co-administered with DNA-HIV-PT123 at weeks (W) 0, 4, and 24. Safety and immunogenicity were monitored until W48. The primary safety endpoint was the proportion of participants per dose cohort and randomized arm without any grade 3 or 4 biological (abnormal laboratory values), or clinical local or systemic solicited, or unsolicited adverse events between W0 and W48 considered to be related or possibly related to the investigational products.
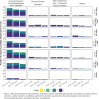
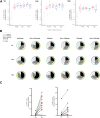
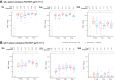
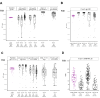
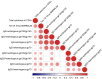
Findings: CD40.HIVRI.Env was well tolerated. Env-specific CD4+ T-cells (IL-2+ or IFN-γ+ or TNF+) were detected in all vaccinees from W6 to W26 and persisted until W48 without a dose-response signal or an effect of DNA-HIV-PT123 co-administration. At W26, IgG response rates (RR) against autologous and nine heterologous gp120/gp140 were 89-100% across all groups and 56-100% at W48. RR against 96ZM651gp70V1V2 were high (90-100%) at W6 and W26 in all groups. Tier1A MW965.26 neutralizing antibody (nAb) titres were detectable in 50-100% of vaccinated individuals at W26, with a dose-response signal, while one volunteer developed nAbs against five Tier2 viruses.
Interpretation: CD40.HIVRI.Env alone or administered with DNA-HIV-PT123 was safe and induced early, and sustained anti-Env cellular and V1V2 IgG responses, identified as correlates of protection in the RV144 trial. CD40 targeting Env-based vaccines may be instrumental for inducing protective vaccine responses in prime-boost strategies.
Funding: ANRS Emerging infectious diseases (ANRS MIE); Vaccine Research Institute (VRI).
Keywords: DNA vaccine; Dendritic cell targeting; First-in-human clinical trial; HIV vaccines; Immunogenicity.
© 2024 The Authors.
Conflict of interest statement
The authors S.Z., G.Z., and Y.L., are named inventors on patent applications based on this work held by Inserm Transfert. J-D.L. declares having received remuneration by the Global HIV Vaccine Enterprise. O.L. declares receipt of grants, consulting fees and support for attending meetings and/or travel from several vaccine industries. She also participated in Data Safety Monitoring or Advisory Boards of Sanofi and MSS. The remaining authors declare no competing interests. Inserm Transfert provided a license for CD40 targeting vaccines to the biotech company LinKinVax.
Figures



References
-
- UNAIDS Fact sheet – latest global and regional statistics on the status of the AIDS epidemic. https://www.unaids.org/sites/default/files/media_asset/UNAIDS_FactSheet Available from:
-
- Rerks-Ngarm S., Pitisuttithum P., Nitayaphan S., et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361(23):2209–2220. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

