Financial implications of treating nonsevere gram-negative clinical mastitis in 3 California dairies
- PMID: 39650003
- PMCID: PMC11624342
- DOI: 10.3168/jdsc.2024-0548
Financial implications of treating nonsevere gram-negative clinical mastitis in 3 California dairies
Abstract
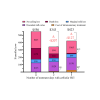
On 3 large California dairies, 415 lactating cows with nonsevere clinical mastitis (CM) and infected with gram-negative (GN) bacteria were randomly assigned to 1 of 3 treatment groups: nontreated control (CON; 135 cases), 2 d of ceftiofur HCl (SP2; 133 cases), or 5 d of ceftiofur HCl (SP5; 147 cases). Bacteriological cure, clinical cure, mastitis recurrence, culling or death, and overall treatment success differed among treatment groups. Although duration of milk withheld due to mastitis therapy was higher for SP5 (9.4 d), there was no difference between CON (6.9 d) and SP2 (7.1 d). Culling and death rates due to GN CM were the main effects that affected partial cost calculations. Of study cows culled across the 3 herds, a higher proportion of CON cows (25%) were culled compared with SP2 (11%) or SP5 (18%). Mastitis-related expenses were higher ($550) for CON than SP2 ($343) or SP5 ($423). Results of this partial budget evaluation for the 3 California dairies indicated economic justification for treating cases of nonsevere GN CM with ceftiofur HCl for 2 d.
© 2024.
Figures
References
-
- Bruno D.R., Cleale R.M., Jardon G., Short T., Mills B., Pedraza J.R. Outcomes after treatment of nonsevere gram-negative clinical mastitis with ceftiofur hydrochloride for 2 or 5 days compared to negative control. J. Dairy Sci. 2024;107:2390–2405. doi: 10.3168/jds.2023-23684. 37923203. - DOI - PubMed
-
- Cha E., Bar D., Hertl J.A., Tauer L.W., Bennett G., González R.N., Schukken Y.H., Welcome F.L., Gröhn Y.T. The cost and management of different types of clinical mastitis in dairy cows estimated by dynamic programming. J. Dairy Sci. 2011;94:4476–4487. doi: 10.3168/jds.2010-4123. 21854920. - DOI - PubMed
-
- de Jong E., Creytens L., De Vliegher S., McCubbin K.D., Baptiste M., Leung A.A., Speksnijder D., Dufour S., Middleton J.R., Ruegg P.L., Lam T.J.G.M., Kelton D.F., McDougall S., Godden S.M., Lago A., Rajala-Schultz P.J., Orsel K., Krömker V., Kastelic J.P., Barkema H.W. Selective treatment of nonsevere clinical mastitis does not adversely affect cure, somatic cell count, milk yield, recurrence, or culling: A systematic review and meta-analysis. J. Dairy Sci. 2023;106:1267–1286. doi: 10.3168/jds.2022-22271. 36543640. - DOI - PubMed
LinkOut - more resources
Full Text Sources

