SLNP-based CDK4- targeted nanotherapy against glioblastoma
- PMID: 39650055
- PMCID: PMC11621005
- DOI: 10.3389/fonc.2024.1455816
SLNP-based CDK4- targeted nanotherapy against glioblastoma
Abstract
Introduction: Glioblastoma is a grade IV solid brain tumor and has a 15-month survival rate even after treatment. Glioblastoma development is heavily influenced by retinoblastoma protein (pRB) pathway changes. The blood-brain barrier, drug resistance, and severe toxicity of Temozolamide are key obstacles in treating glioblastoma. Innovative treatments targeting the pRB pathway with efficient delivery vehicles are required to treat glioblastoma.
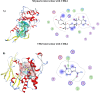
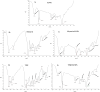
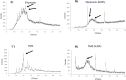
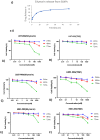
Methods: For this purpose, a library of 691 plant extracts previously tested in vitro for anti-cancerous, anti inflammatory, and anti-proliferative characteristics was created after thorough literature investigations. Compounds were docked against pRB pathway protein ligands using molecular operating environment and chimera. Their nuclear structure and drug-like properties were predicted through Lipinski rule and density functional theory analysis. Physio-chemical characterizations of naked and drug-encapsulated SLNPs assessed size, stability, entrapment efficiency, and drug release rate. Anti-cancer potential of drug and drug- loaded SLNPs was evaluated using U87, U251, and HEK cell lines. Formulations were tested for cancer cell metastatic potential using cell migration assays.
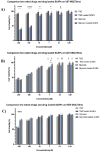
Results: Silymarin (Sil) was identified as the most potent compound against CDK4, which was then encapsulated in stearic acid solid lipid nanoparticles (SLNP-Sil). Sil showed decreased cell viability 72 h after treatment against both U87 and U251 cell lines but had negligible cytotoxic effect on HEK-293. IC50 value of Sil was 155.14 µM for U87 and 195.93 µM for U251. Sil and SLNP-Sil effectively inhibited U87 and U251 cell migration 24 h after treatment.
Discussion: Our results indicated that Sil and SLNP-Sil are promising therapeutic approaches against glioblastoma and merit in vivo experimental verification using orthotropic xenograft mouse models against glioblastoma.
Keywords: blood brain barrier; glioblastoma; silymarin; solid lipid nanoparticles; temozolamide.
Copyright © 2024 Ghani, Ghori, Qamar, Khan, Azad, Habib, Justin, Khan, Shah, Shazly, Bourhia, Perveen and Javed.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
-
- Richterová R, Kolarovszki B. Genetic alterations of glioblastoma. Neurooncol Newer Dev. (2016). doi: 10.5772/63127 - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

