Safety and effectiveness of methylphenidate ER multi-unit pellet system in ADHD patients: An open label study
- PMID: 39650201
- PMCID: PMC11622117
- DOI: 10.4102/sajpsychiatry.v30i0.2267
Safety and effectiveness of methylphenidate ER multi-unit pellet system in ADHD patients: An open label study
Abstract
Background: Attention deficit hyperactive disorder (ADHD) is a neurodevelopmental disorder occurring in children and adults. Pharmacotherapy remains the cornerstone of ADHD treatment. Stimulants such as methylphenidate are effective and have been one of the best studied and most frequently used treatment for ADHD. However, different delivery mechanisms and devices may potentially impact patient experience and real-life outcomes.
Aim: This study evaluated the effectiveness of Multiple-Unit Pellet System Delivered Extended-Release Methylphenidate (Contramyl XR) on symptom control and reported outcomes in ADHD patients, in a real-world setting.
Setting: A phase IV, open label, flexible dose, prospective, observational study conducted at six sites covering five provinces of South Africa.
Methods: About 119 participants with ADHD (both newly diagnosed [treatment-naïve] and methylphenidate-treated [switch-over] patients) were enrolled and initiated either on Contramyl XR or switched over from methylphenidate to Contramyl XR. Primary efficacy was assessed by Weiss Functional Impairment Rating Scale (WFIRS) over 12 weeks.
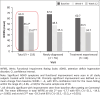
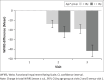
Results: In all, 117 participants completed the study (treatment-naïve patients: 46% [n = 55] and switch-over patients: 54% [n = 64]). Mean change from baseline in total WFIRS (95% confidence interval) was -17.7 (-21.1, -14.3; p < 0.001) at week 4 and -29.3 (-33.5, -25.2; p < 0.001) at week 12. At week 12, there was significant improvement in WFIRS scores, with treatment satisfaction reported by treatment-naïve patients. Switch-over patients also demonstrated comparable effectiveness.
Conclusion: Contramyl XR was found to be clinically effective either as de novo or as switch therapy. It was well tolerated, and all patients chose to continue with the treatment option.
Contribution: Despite distinct and different delivery mechanism of Contramyl XR, this study provides evidence for using it as an alternate treatment option versus reference methylphenidate, in both treatment-naïve and switch-over ADHD patients. Study participants willingness to continue Contramyl XR therapy post study, further strengthens the confidence on the effectiveness of Contramyl XR in managing ADHD patients.
Keywords: Contramyl XR; Weiss Functional Impairment Rating Scale; attention deficit hyperactive disorder; effectiveness; methylphenidate; multiple-unit pellet system; treatment experienced; treatment naïve.
© 2024. The Authors.
Conflict of interest statement
The authors declare that they have no financial or personal relationships that may have inappropriately influenced them in writing this article.
Figures


References
-
- Schellack N, Meyer H. The management of attention deficit-hyperactivity disorder in children. S Afr Pharmaceut J. 2012;79(10):12–20.
-
- Schellack N, Meyer JC. The management of attention deficit/hyperactivity disorder in children: Updated. S Afr Pharmaceut J. 2016;83(4):21–29.
-
- Fisher AJ, Hawkridge S. Attention deficit hyperactivity disorder in children and adolescents. S Afr J Psychiatr. 2013;19(3):136–140. 10.4102/sajpsychiatry.v19i3.943 - DOI
LinkOut - more resources
Full Text Sources
