Engineered myeloid precursors differentiate into osteoclasts and resorb heterotopic ossification in mice
- PMID: 39650237
- PMCID: PMC11620886
- DOI: 10.3389/fbioe.2024.1491962
Engineered myeloid precursors differentiate into osteoclasts and resorb heterotopic ossification in mice
Abstract
Introduction: Heterotopic ossification (HO) occurs following orthopedic trauma, spinal cord injuries, brain trauma and limb amputations. Once symptomatic, HO causes pain, limited mobility and decreased quality of life. Current treatments are limited and have significant complications with high recurrence rates, underscoring the need for improved therapeutic interventions. Osteoclasts (OCs) are physiological bone resorptive cells that secrete enzymes and protons to degrade bone.
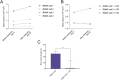
Methods: In this study, we describe the use of genetically engineered OCs as a novel cell therapy approach to treat HO. Inducible, engineered myeloid precursors (iRANK cells) treated with a chemical inducer of dimerization (CID) differentiated into TRAP+ multinucleated OCs and resorbed mineralized tissues in vitro.
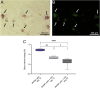
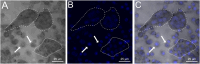
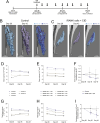
Results: In vivo, BMP-2-induced murine HO lesions were significantly regressed following treatment using iRANK cells with concomitant systemic administration of CID. Moreover, many OCs were TRAP+, MMP9+, and GFP+, indicating that they differentiated from delivered iRANK cells.
Discussion: In summary, these data con rm the ability of engineered myeloid precursors to differentiate into OCs and resorb HO in vivo paving the way for OC delivery as a promising approach for HO treatment.
Keywords: RANK; bone; chemical inducer of dimerization; engineered osteoclasts; heterotopic ossification; osteoclasts; resorption.
Copyright © 2024 Rementer, Yavirach, Buranaphatthana, Walczak, Speer, Pierce, Dharmarajan, Leber, Sangiorzan, Bain, Scatena, Blümke and Giachelli.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

