High-fidelity and iterative affinity extraction of hyaluronan
- PMID: 39650564
- PMCID: PMC11623434
- DOI: 10.1002/pgr2.70008
High-fidelity and iterative affinity extraction of hyaluronan
Abstract
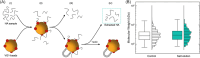
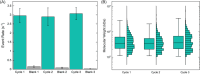
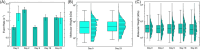
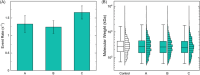
The glycosaminoglycan hyaluronan (HA) serves a variety of crucial physiological functions in vertebrates. Synthesized at the plasma membrane and secreted into the extracellular environment, HA polymers span a wide range of molecular weights (MW) that define their activity through a notable size-function relationship. Analytical technologies for determining HA MW distributions typically require selective extraction from complex biofluids or tissues. A common method for achieving this is immunoprecipitation-like pull-down using specific HA-binding proteins bound to magnetic beads. Here, we present a systematic investigation of experimental variables involved in this process, leading to an affinity extraction protocol that enables iterative bead reuse and reagent lifetime maximization, thereby enhancing the efficiency of the HA extraction process. Our methods provide a framework for general optimization of immunoprecipitation in other contexts with heterogenous analyte sizes.
Keywords: biomarker; hyaluronan; microfluidics; nanopore; nanosensing.
© 2024 The Author(s). Proteoglycan Research published by Wiley Periodicals LLC.
Conflict of interest statement
A.R.H, P.L.D, and E.R are listed as inventors on a patent covering SSNP analysis of HA.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
