G. vaginalis increases HSV-2 infection by decreasing vaginal barrier integrity and increasing inflammation in vivo
- PMID: 39650661
- PMCID: PMC11621107
- DOI: 10.3389/fimmu.2024.1487726
G. vaginalis increases HSV-2 infection by decreasing vaginal barrier integrity and increasing inflammation in vivo
Abstract
Introduction: Clinically, a dysbiotic vaginal microbiota (VMB) colonized with anaerobic species such as Gardnerella vaginalis has been linked to increased susceptibility to viral sexually transmitted infections (STIs) such as Herpes Simplex Virus Type 2 (HSV-2). The mechanism is poorly understood due to the lack of small animal models.
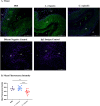
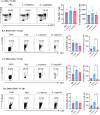
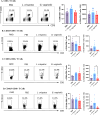
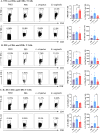
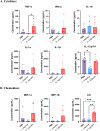
Methods: Mice were inoculated with 107 CFU of the eubiotic bacteria Lactobacillus crispatus, the dysbiotic bacteria G. vaginalis, or PBS as a negative control every 48 h for ten days. On day ten, mice were inoculated with 105 PFU WT HSV-2 333 and survival, pathology, and viral titers were assessed. To elucidate changes in the vaginal microenvironment following bacterial inoculations, vaginal tissue and washes were collected following ten days of inoculations. To assess barrier integrity, tissue was fixed and stained for the barrier protein Desmoglein-1 (DSG-1). To evaluate the immune microenvironment, tissue was processed for flow cytometry to examine tissue-resident T cells and cytokine production by T cells. Vaginal washes were used for multiplex cytokine/chemokine analysis.
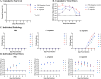
Results: G. vaginalis inoculated mice infected with HSV-2 had significantly decreased survival rates, increased pathology, and higher viral titers than PBS and L. crispatus inoculated mice. The vaginal epithelium of G. vaginalis inoculated mice showed decreased DSG-1 staining compared to other groups, indicating compromised barrier function. Decreased total numbers of CD4+ and CD8+ T cells expressing activated mucosal immune markers CD44, CD69, and CD103 were observed in the vaginal tract of G. vaginalis inoculated mice. They also showed increased proportions of T cells expressing inflammatory cytokines TNF-α and IFN-γ, while L. crispatus inoculated mice had increased proportions and absolute counts of T cells expressing the regulatory cytokine IL-10. In the multiplex assay, vaginal washes from G. vaginalis mice had increased inflammatory cytokines and chemokines compared to L. crispatus and PBS groups.
Discussion: These results suggest G. vaginalis inoculation may be increasing HSV-2 infection by disrupting the epithelial barrier, decreasing protective immune responses and increasing tissue inflammation in the vaginal tract.
Keywords: Lactobacillus; bacterial vaginosis; barrier integrity; female reproductive health; herpes simplex virus; inflammation; mouse models; vaginal microbiota (VMB).
Copyright © 2024 Rahman, Mian, Hayes, Nazli and Kaushic.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Ghartey JP, Smith BC, Chen Z, Buckley N, Lo Y, Ratner AJ, et al. . Lactobacillus crispatus dominant vaginal microbiome is associated with inhibitory activity of female genital tract secretions against escherichia coli. PloS One. (2014) 9:e96659. doi: 10.1371/journal.pone.0096659 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

