Conversion surgery after gemcitabine and cisplatin plus durvalumab for advanced intrahepatic cholangiocarcinoma: A case report
- PMID: 39650816
- PMCID: PMC11514352
- DOI: 10.12998/wjcc.v12.i34.6721
Conversion surgery after gemcitabine and cisplatin plus durvalumab for advanced intrahepatic cholangiocarcinoma: A case report
Abstract
Background: The combination of immune checkpoint inhibitors and chemotherapy has shown promising results for the treatment of advanced biliary tract cancer (BTC). Based on the results of the TOPAZ-1 trial, a gemcitabine and cisplatin plus durvalumab (GCD) regimen was recently approved as first-line therapy for patients with advanced BTC. However, post-GCD conversion surgery has not been previously studied. Herein, we describe a case of advanced intrahepatic cholangiocarcinoma (ICC) successfully treated with radical surgery after GCD.
Case summary: A 65-year-old female diagnosed with advanced ICC with periductal infiltration into the hepatic hilum underwent eight cycles of GCD, followed by durvalumab maintenance treatment, with mild adverse events. Partial response was obtained. Subsequently, a conversion surgery with extended left hepatectomy and bile duct resection was performed. The resection margins were negative, and the pathological diagnosis was compatible with small duct type ICC. The patient remained disease-free for 8 months without adjuvant chemotherapy.
Conclusion: We describe the case of a patient who received successful conversion surgery after GCD treatment for advanced ICC.
Keywords: Case report; Conversion surgery; Immunotherapy; Intrahepatic cholangiocarcinoma; Small duct type.
©The Author(s) 2024. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: The authors declare that they have no conflict of interest to disclose.
Figures
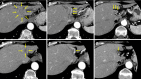
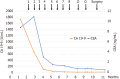

References
-
- Oh DY, Ruth He A, Qin S, Chen LT, Okusaka T, Vogel A, Kim JW, Suksombooncharoen T, Ah Lee M, Kitano M, Burris H, Bouattour M, Tanasanvimon S, McNamara MG, Zaucha R, Avallone A, Tan B, Cundom J, Lee CK, Takahashi H, Ikeda M, Chen JS, Wang J, Makowsky M, Rokutanda N, He P, Kurland JF, Cohen G, Valle JW. Durvalumab plus Gemcitabine and Cisplatin in Advanced Biliary Tract Cancer. NEJM Evid. 2022;1:EVIDoa2200015. - PubMed
-
- Brierley JD, Gospodarowicz MK, Wittekind C. Skin Tumours. TNM Online. 2017
-
- National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) v5.0. CTEP 2017. Available from: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc..... [Accessed March 29, 2024]
-
- Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

