Hydrates of N-((10-Chloroanthracen-9-yl)methyl)-3-(1H-imidazol-1-yl)propan-1-ammonium Cobalt(II), Copper(II), and Zinc(II) 2,6-Pyridinedicarboxylate: Reversible Crystallization
- PMID: 39651092
- PMCID: PMC11618415
- DOI: 10.1021/acsomega.4c08822
Hydrates of N-((10-Chloroanthracen-9-yl)methyl)-3-(1H-imidazol-1-yl)propan-1-ammonium Cobalt(II), Copper(II), and Zinc(II) 2,6-Pyridinedicarboxylate: Reversible Crystallization
Abstract
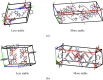
In a quest to explore interconvertible assemblies of hydrates of cobalt(II), copper(II), and zinc(II) 2,6-pyridinedicarboxylate (26-pdc), complexes having cation of a chloro-substituted analogue N-{(10-chloroanthracen-9-yl)methyl}-3-(1H-imidazol-1-yl)propan-1-amine were investigated. In the case of cobalt and copper complexes, a crystallized stable hydrate and a less stable methanol hydrate were guided by concentration-dependent crystallizations. The unit-cells of the crystals of the methanol hydrates of the two cobalt and copper complexes each belong to the P1̅ space group but have different stoichiometries as well as large differences in packing. These hydrates could be reversibly crystallized in a predictable manner. The unit-cell volumes of the methanol hydrate of the cobalt complex were four-times smaller than that of the respective stable form (C2/c space group), whereas similar hydrates of the copper complex had a two-times smaller unit-cell volume than that of the stable form. The cations of the stable forms assembled together and formed zigzag ladder-like chains. The spaces present in between the assembled chains were filled with clusters of face to face stacked anions. The transformation to stable form required a bottom-up building process of the unit-cell starting from a smaller unit-cell of the less stable hydrates. Fluorescence spectroscopic studies showed the possibility of two forms of assemblies of the zinc-complex in solution, but crystallization had yielded only the stable form.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures







References
-
- A reversible reaction of hydrated copper(II) sulfate; Royal Society of Chemistry, 2024. https://edu.rsc.org/experiments/a-reversible-reaction-of-hydrated-copper....
-
- The equilibrium between two coloured cobalt species; Royal Society of Chemistry, 2024. https://edu.rsc.org/experiments/the-equilibrium-between-two-coloured-cob....
-
- Wells A. F. The crystal structures of salt hydrates and complex halides. Q. Rev. Chem. Soc. 1954, 8, 380–403. 10.1039/qr9540800380. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous
