Cancer Cells Show Higher Sensitivity to Melatonin-Tamoxifen Drug Conjugates than to Combination of Melatonin and Tamoxifen
- PMID: 39651096
- PMCID: PMC11618438
- DOI: 10.1021/acsomega.4c08881
Cancer Cells Show Higher Sensitivity to Melatonin-Tamoxifen Drug Conjugates than to Combination of Melatonin and Tamoxifen
Abstract
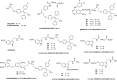
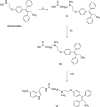
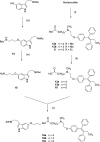
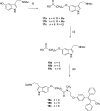
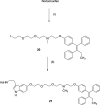
Drug conjugates of tamoxifen and melatonin linked through the amide side chain of melatonin (4a,4b) were reported as promising agents for future treatment of breast cancer, possibly reversing the adverse effects of tamoxifen. Here, we report the synthesis and pharmacological evaluation of a novel series of anticancer drug conjugates linking melatonin with tamoxifen through polymethylene spacers through the ether oxygen of melatonin (16a-c, 19a-c, 21) and compare them to the previously reported amide-linked analogues 4a and 4b. All hybrid ligands are antagonists of estrogen receptor alpha and agonists of the melatonin MT1 receptor with variable potencies. Several drug conjugates including the (CH2)4-linked analogues 4a and 16a and the (CH2)6-linked compound 16c showed higher potency to inhibit cell viability than the combination of melatonin and tamoxifen on at least one cancer cell line including MCF-7, MDA-MB-231, and HT-1080.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
-
- Singh A. K.; Kumar A.; Singh H.; Sonawane P.; Paliwal H.; Thareja S.; Pathak P.; Grishina M.; Jaremko M.; Emwas A. H.; Yadav J. P.; Verma A.; Khalilullah H.; Kumar P. Concept of Hybrid Drugs and Recent Advancements in Anticancer Hybrids. Pharmaceuticals 2022, 15 (9), 1071. 10.3390/ph15091071. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
