This is a preprint.
TTF2 promotes replisome eviction from stalled forks in mitosis
- PMID: 39651145
- PMCID: PMC11623681
- DOI: 10.1101/2024.11.30.626186
TTF2 promotes replisome eviction from stalled forks in mitosis
Abstract
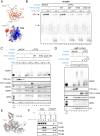
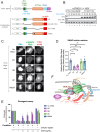
When cells enter mitosis with under-replicated DNA, sister chromosome segregation is compromised, which can lead to massive genome instability. The replisome-associated E3 ubiquitin ligase TRAIP mitigates this threat by ubiquitylating the CMG helicase in mitosis, leading to disassembly of stalled replisomes, fork cleavage, and restoration of chromosome structure by alternative end-joining. Here, we show that replisome disassembly requires TRAIP phosphorylation by the mitotic Cyclin B-CDK1 kinase, as well as TTF2, a SWI/SNF ATPase previously implicated in the eviction of RNA polymerase from mitotic chromosomes. We find that TTF2 tethers TRAIP to replisomes using an N-terminal Zinc finger that binds to phosphorylated TRAIP and an adjacent TTF2 peptide that contacts the CMG-associated leading strand DNA polymerase ε. This TRAIP-TTF2-pol ε bridge, which forms independently of the TTF2 ATPase domain, is essential to promote CMG unloading and stalled fork breakage. Conversely, RNAPII eviction from mitotic chromosomes requires the ATPase activity of TTF2. We conclude that in mitosis, replisomes undergo a CDK- and TTF2-dependent structural reorganization that underlies the cellular response to incompletely replicated DNA.
Conflict of interest statement
Competing interests: J.C.W. is a co-founder of MOMA therapeutics, in which he has a financial interest. D.P. is on the scientific advisory board of Volastra Therapeutics.
Figures



References
-
- West S. C., Blanco M. G., Chan Y. W., Matos J., Sarbajna S., Wyatt H. D. M., Resolution of Recombination Intermediates: Mechanisms and Regulation. Cold Spring Harb. Symp. Quant. Biol. 80, 103–109 (2015). - PubMed
-
- Tsherniak A., Vazquez F., Montgomery P. G., Weir B. A., Kryukov G., Cowley G. S., Gill S., Harrington W. F., Pantel S., Krill-Burger J. M., Meyers R. M., Ali L., Goodale A., Lee Y., Jiang G., Hsiao J., Gerath W. F. J., Howell S., Merkel E., Ghandi M., Garraway L. A., Root D. E., Golub T. R., Boehm J. S., Hahn W. C., Defining a Cancer Dependency Map. Cell 170, 564–576.e16 (2017). - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
