This is a preprint.
Hippocampal CA2 neurons disproportionately express AAV-delivered genetic cargo
- PMID: 39651273
- PMCID: PMC11623684
- DOI: 10.1101/2024.11.27.625768
Hippocampal CA2 neurons disproportionately express AAV-delivered genetic cargo
Abstract
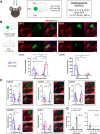
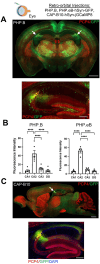
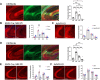
Hippocampal area CA2 is unique in many ways, largely based on the complement of genes expressed there. We and others have observed that CA2 neurons exhibit a uniquely robust tropism for adeno-associated viruses (AAVs) of multiple serotypes and variants. In this study, we aimed to systematically investigate the propensity for AAV tropism toward CA2 across a wide range of AAV serotypes and variants, injected either intrahippocampally or systemically, including AAV1, 2, 5, 6, 8, 9, DJ, PHP.B, PHP.eB, and CAP-B10. We found that most serotypes and variants produced disproportionally high expression of AAV-delivered genetic material in hippocampal area CA2, although two serotypes (AAV6 and DJ) did not. In an effort to understand the mechanism(s) behind this observation, we considered perineuronal nets (PNNs) that ensheathe CA2 pyramidal cells and, among other functions, buffer diffusion of ions and molecules. We hypothesized that PNNs might attract AAV particles and maintain them in close proximity to CA2 neurons, thereby increasing exposure to AAV particles. However, genetic deletion of PNNs from CA2 had no effect on AAV transduction. Next, we next considered the AAV binding factors and receptors known to contribute to AAV transduction. We found that the AAV receptor (AAVR), which is critical to transduction, is abundantly expressed in CA2, and knockout of AAVR nearly abolished expression of AAV-delivered material by all serotypes tested. Additionally, we found CA2 enrichment of several cell-surface glycan receptors that AAV particles attach to before interacting with AAVR, including heparan sulfate proteoglycans, N-linked sialic acid and N-linked galactose. Indeed, CA2 showed the highest expression of AAVR and the investigated glycan receptors within the hippocampus. We conclude that CA2 neurons are endowed with multiple factors that make it highly susceptible to AAV transduction, particularly to the systemically available PHP variants, including CAP-B10. Given the curved structure of hippocampus and the relatively small size of CA2, systemic delivery of engineered PHP or CAP variants could all but eliminate the need for intrahippocampal AAV injections, particularly when injecting recombinase-dependent AAVs into animals that express recombinases in CA2.
Conflict of interest statement
Conflict of Interest Statement: The authors declare no competing financial interests.
Figures





References
-
- Bai X, Brown JR, Varki A, Esko JD (2001) Enhanced 3-O-sulfation of galactose in Asn-linked glycans and Maackia amurenesis lectin binding in a new Chinese hamster ovary cell line. Glycobiology 11:621–632. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
