This is a preprint.
Structural and functional insights into activation and regulation of the dynein-dynactin-NuMA complex
- PMID: 39651296
- PMCID: PMC11623564
- DOI: 10.1101/2024.11.26.625568
Structural and functional insights into activation and regulation of the dynein-dynactin-NuMA complex
Abstract
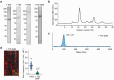
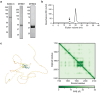
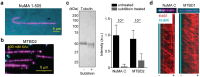
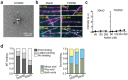
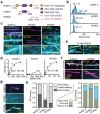
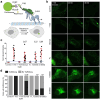
During cell division, NuMA orchestrates the focusing of microtubule minus-ends in spindle poles and cortical force generation on astral microtubules by interacting with dynein motors, microtubules, and other cellular factors. Here we used in vitro reconstitution, cryo-electron microscopy, and live cell imaging to understand the mechanism and regulation of NuMA. We determined the structure of the processive dynein/dynactin/NuMA complex (DDN) and showed that the NuMA N-terminus drives dynein motility in vitro and facilitates dynein-mediated transport in live cells. The C-terminus of NuMA directly binds to and suppresses the dynamics of the microtubule minus-end. Full-length NuMA is autoinhibited, but mitotically phosphorylated NuMA activates dynein in vitro and interphase cells. Together with dynein, activated full-length NuMA focuses microtubule minus-ends into aster-like structures. The binding of the cortical protein LGN to the NuMA C-terminus results in preferential binding of NuMA to the microtubule plus-end. These results provide critical insights into the activation of NuMA and dynein for their functions in the spindle body and the cell cortex.
Figures











References
-
- Merdes A., Ramyar K., Vechio J.D. & Cleveland D.W. A complex of NuMA and cytoplasmic dynein is essential for mitotic spindle assembly. Cell 87, 447–458 (1996). - PubMed
-
- Heald R. et al. Self-organization of microtubules into bipolar spindles around artificial chromosomes in Xenopus egg extracts. Nature 382, 420–425 (1996). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
