CNS B cell infiltration in tumefactive anti-myelin oligodendrocyte glycoprotein antibody-associated disease
- PMID: 39651331
- PMCID: PMC11622319
- DOI: 10.1177/20552173241301011
CNS B cell infiltration in tumefactive anti-myelin oligodendrocyte glycoprotein antibody-associated disease
Abstract
Background: Few studies have examined B cells among patients with anti-myelin oligodendrocyte glycoprotein (MOG) antibody-associated disease (MOGAD), including brain pathology.
Objective: To describe cases of tumefactive MOGAD with B-cell dominant central nervous system (CNS) infiltration.
Methods: In this study, we reviewed three cases with clinical and brain histopathological features with tumefactive MOGAD.
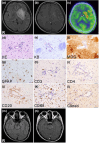
Results: Forty-nine cases of tumefactive brain lesions (TBL) between January 2003 and December 2023 were included; of these, seven had MOGAD. Three underwent a brain biopsy. B-cell dominant CNS infiltration was observed in two cases. In two cases with B-cell dominant CNS infiltration, symptoms included fever, headache, nausea, somnolence, and focal neurological deficits. Cerebrospinal fluid examination revealed both mild pleocytosis and negative oligoclonal IgG bands. Magnetic resonance imaging of the brain revealed large abnormal lesions extending from the basal ganglia to the parietotemporal lobe in both cases. These cases showed a good response to steroids; however, one case relapsed. Brain pathology showed demyelination and perivascular lymphocytic infiltration. One showed small vessel vasculitis. Deposition of the activated complement component was absent or rarely observed. Loss of MOG was observed in two cases.
Conclusion: MOGAD could exhibit B-cell dominant CNS infiltration and small vessel vasculitis. MOGAD should be considered in differential diagnosis of TBL.
Keywords: Anti-myelin oligodendrocyte glycoprotein antibody-associated disease; B cell; brain histopathology; brain tumor; myelin oligodendrocyte glycoprotein; tumefactive brain lesion.
© The Author(s), 2024.
Conflict of interest statement
RI received honoraria for talks from Alexion Pharmaceuticals, Biogen Idec Japan, Chugai Pharmaceutical Co., Ltd., and Novartis Pharma. TM received speaker honoraria from Tanabe Mitsubishi Pharma, Chugai Pharma, Novartis Pharma, Alexion Pharma, Teijin Pharma, Viela Bio, and Biogen Idec Japan, and received research support from Cosmic Corporation and Medical and Biological Laboratories Co.; and received Grant-in-Aid for scientific research from Ministry of Education, Culture, Sports, Science, and Technology. YS received honoraria for speakers from Alexion Pharmaceuticals, Novartis Pharma, Biogen Idec Japan, and Chugai Pharmaceutical Co., Ltd.
Figures


References
-
- Johns TG, Bernard CC. The structure and function of myelin oligodendrocyte glycoprotein. J Neurochem 1999; 72: 1–9. - PubMed
-
- Banwell B, Bennett JL, Marignier R, et al. Diagnosis of myelin oligodendrocyte glycoprotein antibody-associated disease: international MOGAD panel proposed criteria. Lancet Neurol 2023; 22: 268–282. - PubMed
-
- Takai Y, Misu T, Kaneko K, et al. Myelin oligodendrocyte glycoprotein antibody-associated disease: an immunopathological study. Brain 2020; 143: 1431–1446. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

