Bioleaching of lithium from jadarite, spodumene, and lepidolite using Acidiothiobacillus ferrooxidans
- PMID: 39651346
- PMCID: PMC11622194
- DOI: 10.3389/fmicb.2024.1467408
Bioleaching of lithium from jadarite, spodumene, and lepidolite using Acidiothiobacillus ferrooxidans
Abstract
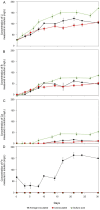
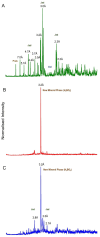
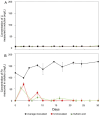
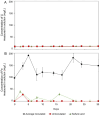
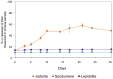
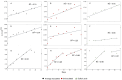
Lithium (Li) is becoming increasingly important due to its use in clean technologies that are required for the transition to net zero. Although acidophilic bioleaching has been used to recover metals from a wide range of deposits, its potential to recover Li has not yet been fully explored. In this study, we used a model Fe(II)- and S-oxidising bacterium, Acidiothiobacillus ferrooxidans (At. Ferrooxidans), to extract Li from three different minerals and kinetic modelling to predict the dominant reaction pathways for Li release. Bioleaching of Li from the aluminosilicate minerals lepidolite (K(Li,Al)3(Al,Si,Rb)4O10(F,OH)2) and spodumene (LiAl(Si2O6)) was slow, with only up to 14% (approximately 12 mg/L) of Li released over 30 days. By contrast, At. ferrooxidans accelerated Li leaching from a Li-bearing borosilicate clay (jadarite, LiNaB3SiO7OH) by over 50% (over 120 mg/L) in 21 days of leaching, and consistently enhanced Li release throughout the experiment compared to the uninoculated control. Biofilm formation and flocculation of sediment occurred exclusively in the experiments with At. ferrooxidans and jadarite. Fe(II) present in the jadarite-bearing clay acted as an electron donor. Chemical leaching of Li from jadarite using H2SO4 was most effective, releasing approximately 75% (180 mg/L) of Li, but required more acid than bioleaching for pH control. Kinetic modelling was unable to replicate the data for jadarite bioleaching after primary abiotic leaching stages, suggesting additional processes beyond chemical leaching were responsible for the release of Li. A new crystalline phase, tentatively identified as boric acid, was observed to form after acid leaching of jadarite. Overall, the results demonstrate the potential for acidophilic bioleaching to recover Li from jadarite, with relevance for other Li-bearing deposits.
Keywords: bioleaching; critical minerals; iron bio-oxidation; lithium; metal recovery.
Copyright © 2024 Kirk, Newsome, Falagan and Hudson-Edwards.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures

References
-
- Alavia W., Seidel-Morgenstern A., Hermsdorf D., Lorenz H., Graber T. A. (2023). Real-time crystal growth monitoring of boric acid from sodium or Lithium sulfate containing aqueous solutions by atomic force microscopy. ACS Omega 8, 10822–10835. doi: 10.1021/acsomega.2c06953, PMID: - DOI - PMC - PubMed
-
- Ata O., Çolak S., Çopur M., Çelik C. (2000). Determination of the optimum conditions for boric acid extraction with carbon dioxide gas in Aqueous Media from Colemanite Containing Arsenic. Ind. Eng. Chem. Res. 39, 488–493. doi: 10.1021/ie990314z - DOI
-
- Bahaloo-Horeh N., Mousavi S. M., Baniasadi M. (2018). Use of adapted metal tolerant aspergillus Niger to enhance bioleaching efficiency of valuable metals from spent lithium-ion mobile phone batteries. J. Clean. Prod. 197, 1546–1557. doi: 10.1016/j.jclepro.2018.06.299 - DOI
-
- Bradley D. C., McCauley A. D., Stillings L. M. (2010). Mineral-deposit model for lithium-cesium-tantalum pegmatites: U.S. Geological Survey Scientific Investigations Report 2010–5070–O, 48. doi: 10.3133/sir20105070O - DOI
-
- Bradley D., Stillings L. L., Jaskula B. W., Munk L. A., McCauley A. D. (2017). Lithium, Eds. K. Chap, K. J. Schulz, J. H. DeYoung, Jr., R. R. Seal, II, and D. C. Bradley. Critical mineral resources of the United States—Economic and environmental geology and prospects for future supply: U.S. Geological Survey professional paper 1802.
LinkOut - more resources
Full Text Sources

