MinD proteins regulate CetZ1 localization in Haloferax volcanii
- PMID: 39651350
- PMCID: PMC11621097
- DOI: 10.3389/fmicb.2024.1474697
MinD proteins regulate CetZ1 localization in Haloferax volcanii
Abstract
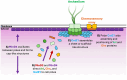
CetZ proteins are archaea-specific homologs of the cytoskeletal proteins FtsZ and tubulin. In the pleomorphic archaeon Haloferax volcanii, CetZ1 contributes to the development of rod shape and motility, and has been implicated in the proper assembly and positioning of the archaellum and chemotaxis motility proteins. CetZ1 shows complex subcellular localization, including irregular midcell structures and filaments along the long axis of developing rods and patches at the cell poles of the motile rod cell type. The polar localizations of archaellum and chemotaxis proteins are also influenced by MinD4, the only previously characterized archaeal member of the MinD family of ATPases, which are better known for their roles in the positioning of the division ring in bacteria. Using minD mutant strains and CetZ1 subcellular localization studies, we show here that a second minD homolog, minD2, has a strong influence on motility and the localization of CetZ1. Knockout of the minD2 gene altered the distribution of a fluorescent CetZ1-mTq2 fusion protein in a broad midcell zone and along the edges of rod cells, and inhibited the localization of CetZ1-mTq2 at the cell poles. MinD4 had a similar but weaker influence on motility and CetZ1-mTq2 localization. The minD2/4 mutant strains formed rod cell shapes like the wildtype at an early log stage of growth. Our results are consistent with distinct roles for CetZ1 in rod shape formation and at the poles of mature rods, that are positioned through the action of the MinD proteins and contribute to the development of swimming motility in multiple ways. They represent the first report of MinD proteins controlling the positioning of tubulin superfamily proteins in archaea.
Keywords: archaea; cytoskeleton; halophile; motility; protein localization; tubulin superfamily.
Copyright © 2024 Brown and Duggin.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures

References
LinkOut - more resources
Full Text Sources

