Triplet therapy for metastatic castration-sensitive prostate cancer: Rationale and clinical evidence
- PMID: 39651632
- PMCID: PMC11923528
- DOI: 10.1111/iju.15647
Triplet therapy for metastatic castration-sensitive prostate cancer: Rationale and clinical evidence
Abstract
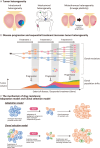
Prostate cancer (PC) growth is hormone-dependent and it frequently develops distant metastases as disease progresses. Patients with metastatic castration-sensitive prostate cancer (mCSPC) initially respond to androgen deprivation therapy (ADT) but eventually become refractory and develop metastatic castration-resistant prostate cancer (mCRPC). Castration-resistance is associated with high lethality and metastases confer poor prognosis, therefore unmet needs in treatment for mCSPC remain high. So far, improvements in survival in mCSPC have been achieved by doublet combination therapy such as docetaxel or an androgen-receptor signaling inhibitor (ARSI) in addition to ADT. Further, recent phase 3 trials have shown that triplet therapy-a combination of ARSI, docetaxel, and ADT improves prognosis compared with docetaxel plus ADT in mCSPC. PC tumors manifest intra- and inter-tumoral heterogeneity at both the genetic and phenotypic level. As heterogeneity increases during sequential treatment and disease progression, it is reasonable to initiate combination therapy using drugs with different mechanisms of action early in the course of disease, such as mCSPC. Previous research about tumor heterogeneity and drug resistant mechanism support this rationale, as well as preclinical studies and real-world data provide the scientific evidence of benefit by combining ARSI and docetaxel. Here, we review the rationale and clinical evidence for triplet therapy in patients with mCSPC.
Keywords: androgen receptor signaling inhibitor; docetaxel; metastatic castration‐sensitive prostate cancer; prostate cancer; triplet therapy.
© 2024 The Author(s). International Journal of Urology published by John Wiley & Sons Australia, Ltd on behalf of The Japanese Urological Association.
Conflict of interest statement
H.S. reported receiving honoraria for lectures from Bayer Yakuhin, Ltd., Astellas Pharma Inc., AstraZeneca K.K., Janssen Pharmaceutical K.K., Ferring Pharmaceuticals Co., Ltd. and Sanofi K.K., and grants or funding from Bayer Yakuhin, Ltd., Astellas Pharma Inc., AstraZeneca K.K., Janssen Pharmaceutical K.K., MSD K.K. and Eli Lilly Japan K.K.
S.A. reported receiving honoraria from Janssen Pharmaceutical K.K., Bayer Yakuhin, Ltd., Sanofi K.K., Takeda Pharmaceutical Co., Ltd., Astellas Pharma Inc. and Pfizer Japan Inc., research grant from Tosoh Co. and advisory board for Janssen Pharmaceutical K.K., Bayer Yakuhin, Ltd., Sanofi K.K., Takeda Pharmaceutical Co., Ltd., Astellas Pharma Inc., Pfizer Japan Inc. and Novartis Pharma K.K.
M.S. reported receiving honoraria for lectures from Janssen Pharmaceutical K.K., Astellas Pharma Inc., AstraZeneca K.K. and Sanofi K.K. and grants or funding from KUBIX Inc. and Astellas Pharma Inc.
H.K. is an employee of Bayer Yakuhin Ltd.
T.K. reported receiving honoraria for lectures from Astellas Pharma Inc., AstraZeneca K.K., Sanofi K.K., Bayer Yakuhin Ltd. and Janssen Pharmaceutical K.K.
Dr. Shusuke Akamatsu is an Associate Editor of the International Journal of Urology. Dr. Hiroyoshi Suzuki and Dr. Takahiro Kimura are Editorial Board members of the International Journal of Urology. They are co‐authors of this article.
Figures

References
-
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. - PubMed
-
- Rebello RJ, Oing C, Knudsen KE, Loeb S, Johnson DC, Reiter RE, et al. Prostate cancer. Nat Rev Dis Primers. 2021;7:9. - PubMed
-
- Fujimoto H, Nakanishi H, Miki T, Kubota Y, Takahashi S, Suzuki K, et al. Oncological outcomes of the prostate cancer patients registered in 2004: report from the cancer registration committee of the JUA. Int J Urol. 2011;18:876–881. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

