Azathioprine for people with multiple sclerosis
- PMID: 39651635
- PMCID: PMC11626701
- DOI: 10.1002/14651858.CD015005.pub2
Azathioprine for people with multiple sclerosis
Abstract
Background: Multiple sclerosis (MS) is an immune-mediated, chronic, inflammatory demyelinating disease of the central nervous system, impacting around 2.8 million people worldwide. Characterised by recurrent relapses or progression, or both, it represents a substantial global health burden, affecting people, predominantly women, at a young age (the mean age of diagnosis is 32 years). Azathioprine is used to treat chronic inflammatory and autoimmune diseases, and it is used in clinical practice as an off-label intervention for MS, especially where access to on-label disease-modifying treatments (DMTs) for MS is limited. Given this, a review of azathioprine's benefits and harms would be timely and valuable to inform shared healthcare decisions.
Objectives: To evaluate the benefits and harms of azathioprine (AZA) for relapsing and progressive multiple sclerosis (MS), compared to other disease-modifying treatments (DMTs), placebo or no treatment. Specifically, we will assess the following comparisons. AZA compared with other DMTs or placebo as first-choice treatment for relapsing forms of multiple sclerosis AZA compared with other DMTs or placebo for relapsing forms of MS when switching from another DMT AZA compared with other DMTs or placebo as first-choice treatment for progressive forms of MS AZA compared with other DMTs or placebo for progressive forms of MS when switching from another DMT SEARCH METHODS: We conducted an extensive search for relevant literature using standard Cochrane search methods. The most recent search date was 9 August 2023.
Selection criteria: We included randomised controlled trials (RCTs) lasting 12 months or more that compared azathioprine versus DMTs, placebo or no intervention in adults with MS. We considered evidence from non-randomised studies of interventions (NRSIs) as these studies may provide additional evidence not available from RCTS. We excluded cluster-randomised trials, cross-over trials, interrupted time series, case reports and studies of within-group design with no control group.
Data collection and analysis: We followed standard Cochrane methodology. There were three outcomes we considered to be critical: disability, relapse and serious adverse events (SAEs, as defined in the studies). We were also interested in other important outcomes: quality-of-life (QoL) impairment (mental score), short-term adverse events (gastrointestinal disorders), long-term adverse events (neoplasms) and mortality.
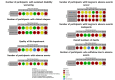
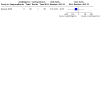
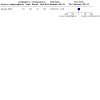
Main results: We included 14 studies: eight RCTs (1076 participants included in meta-analyses) and six NRSIs (1029 participants). These studies involved people with relapsing and progressive MS. Most studies included more women (57 to 83%) than men, with participants' average age at the onset of MS being between 29.4 and 33.4 years. Five RCTs and all six NRSIs were conducted in Europe (1793 participants); two RCTs were conducted in the USA (126 participants) and one in Iran (94 participants). The RCTs lasted two to three years, while NRSIs looked back up to 10 years. Four studies received some funding or support from commercial interests and five were funded by government or philanthropy; the other five provided no information about funding. There are three ongoing studies. Comparison groups included other DMTs (interferon beta and cyclosporine A), placebo or no treatment. Below, we report on azathioprine as a 'first choice' treatment compared to interferon beta for people with relapsing MS. None of the studies reported on any critical or important outcome for this comparison for progressive MS. No study was retrieved comparing azathioprine to placebo or other DMTs for either relapsing or progressive MS. Furthermore, the NRSIs did not provide information not already covered in the RCTs. Azathioprine as a first-choice treatment compared to other DMTs (specifically, interferon beta) for relapsing MS - The evidence is very uncertain about the effect of azathioprine on the number of people with disability progression over two years compared to interferon beta (risk ratio (RR) 0.19, 95% confidence interval (CI) 0.02 to 1.58; 1 RCT, 148 participants; very low certainty evidence). - Azathioprine may decrease the number of people with relapses over a one- to two-year follow-up compared to interferon beta (RR 0.61, 95% CI 0.43 to 0.86; 2 RCTs, 242 participants; low-certainty evidence). - Azathioprine may result in a possible increase in the number of people with SAEs over two years in comparison with interferon beta (RR 6.64, 95% CI 0.35 to 126.27; 1 RCT, 148 participants; low-certainty evidence). - The evidence is very uncertain about the effect of azathioprine on the number of people with the short-term adverse event of gastrointestinal disorders over two years compared to interferon beta (RR 5.30, 95% CI 0.15 to 185.57; 2 RCTs, 242 participants; very low certainty evidence). We found no evidence comparing azathioprine to other DMTs for QoL impairment (mental score), long-term adverse events (neoplasms) or mortality.
Authors' conclusions: Azathioprine has been proposed as an alternative treatment for MS when access to approved, on-label DMTs is limited, especially in resource-limited settings. The limited evidence available suggests that azathioprine may result in a modest benefit in terms of relapse frequency, with a possible increase in SAEs, when compared to interferon beta-1b, for people with relapsing-remitting multiple sclerosis. The evidence for the effect on disability progression and short-term adverse events is very uncertain. Caution is required in interpreting the conclusions of this review since our certainty in the available evidence on the benefits and harms of azathioprine in multiple sclerosis is low to very low, implying that further evidence is likely to change our conclusions. An important limitation we noted in the available evidence is the lack of long-term comparison with other treatments and the failure of most studies to measure outcomes that are important to people with multiple sclerosis, such as quality of life and cognitive decline. This is especially the case in the evidence relevant to people with progressive forms of multiple sclerosis.
Copyright © 2024 The Authors. Cochrane Database of Systematic Reviews published by John Wiley & Sons, Ltd. on behalf of The Cochrane Collaboration.
Conflict of interest statement
FN is Joint Co‐ordinating Editor of the Cochrane Multiple Sclerosis and Rare Disease of the CNS review group. He was not involved in the editorial process of the review. FN works as an Epidemiologist and Neurologist within the Italian Public Health Service, at an outpatient clinic at the IRCCS Instituto delle Scienze Neurologiche di Bologna.
EB is a neurologist, working at an outpatient clinic at the IRCCS Instituto delle Scienze Neurologiche di Bologna. She is an author of a manuscript on ponesimod for the treatment of relapsing multiple sclerosis, and has received travel and meeting attendance support from Biogen, Roche, and Sanofi Genzyme; personal payments.
BR has worked as the Managing Editor of the Cochrane Multiple Sclerosis and Rare Disease of the CNS review group and is a Managing Editor with the Central Editorial Service. He was not involved in the editorial process of the review.
IC works at the IRCCS San Camillo Hospital, Venice, Italy.
GF is Joint Co‐ordinating Editor of the Cochrane Multiple Sclerosis and Rare Disease of the CNS review group; he was not involved in the editorial process of this review. GF was involved with MAIN trial 2014 (Massacesi 2014), funded by the AIFA (Italian Medicines Agency). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript, and GF declares that no competing interests exist. The MAIN trial was approved by ethics committees in the co‐ordinating centre (Careggi University Hospital, Ethic Committee, Florence) and in each of the participating centres (Fondazione IRCCS Istituto Neurologico Carlo Besta, Milano; Clinica Neurologica, Novara; Università 'La Sapienza', Roma; Policlinico 'G Rodolico' Azienda Ospedaliero‐Universitaria, Catania; Clinica Neurologica 2, Genova; Azienda Ospedaliera Universitaria Integrata, Verona; Ospedale Clinicizzato 'Colle Dall’Ara', Chieti; Università di Sassari, Sassari; Università di Napoli, Napoli; Ospedale S Antonio, Padova; Ospedale Civile S Agostino‐Estense, Modena; Ospedale Santa Maria, Reggio Emilia; Policlinico Universitario Mater Domini, Catanzaro; Ospedale S Gerardo, Monza; Azienda Ospedaliero‐Universitaria S Anna, Ferrara; Ospedali Riuniti, Ancona; Istituto S Raffaele 'G. Giglio', Cefalu; Azienda Ospedaliero San Giovanni Battista, Università di Torino, Torino; Ospedale Sacro Cuore, Negrar; Ospedale Santa Chiara, Trento; Ospedale Regionale, Bolzano; Azienda Ospedaliero‐Universitaria Senese, Policlinico 'Le Scotte', Siena; Ospedale 'Misericordia e Dolce', Prato; Università degli Studi di Pisa, Pisa; Policlinico 'G Martino’ Messina; Università degli Studi di Palermo, Palermo; Università Cattolica, Policlinico Gemelli, Roma; Dipartimento Neuroriabilitativo ASL CN1, Cuneo; Luigi Gonzaga Hospital, Orbassano Ethics Committees). The MAIN trial adhered to Good Clinical Practice (GCP) guidelines and Declaration of Helsinki.
GI declares that he has no conflicts of interest. GI is a neurologist. He previously worked in the public health system in Italy and is currently working as neurologist and psychiatrist in private practice.
Figures
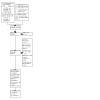

















































Update of
References
References to studies included in this review
Amato 1993 {published data only}
British and Dutch 1988 {published data only}
-
- British and Dutch Multiple Sclerosis Azathioprine Trial Group. Double-masked trial of azathioprine in multiple sclerosis. Lancet 1988;2:179-83. [PMID: ] - PubMed
Confavreux 1996 {published data only}
Ellison 1989 {published data only}
-
- Ellison GW, Myers LW, Mickey ME, Graves MC, Tourtellotte WW, Syndulko, et al. A placebo-controlled, randomized, double-masked, variable dosage, clinical trial of azathioprine with and without methylprednisolone in multiple sclerosis. Neurology 1989;39:1018-26. [DOI: 10.1212/wnl.39.8.1018] - DOI - PubMed
Etemadifar 2007 {published data only}
Goodkin 1991 {published data only}
Havrdova 2009 {published data only}
-
- Horakova D, Cox JL, Havrdova E, Hussein S, Dolezal O, Cookfair D, et al. Evolution of different MRI measures in patients with active relapsing-remitting multiple sclerosis over 2 and 5 years: a case-control study. Journal of Neurology, Neurosurgery and Psychiatry 2008;79(4):407-14. [DOI: 10.1136/jnnp.2007.120378] - DOI - PubMed
-
- Horakova D, Dwyer MG, Havrdova E, Cox JL, Dolezal O, Bergsland N, et al. Gray matter atrophy and disability progression in patients with early relapsing-remitting multiple sclerosis: a 5-year longitudinal study. Journal of the Neurological Sciences 2009;282(1-2):112-9. [DOI: 10.1016/j.jns.2008.12.005] - DOI - PubMed
-
- NCT01628315. Assessment of lesion activity analysis in the avonex-steroid azathioprine (ASA) Study (ASA). https://clinicaltrials.gov/study/NCT01628315 (first submitted 1 May 2012).
Kappos 1988 {published data only}
-
- Haas J, Stark E, Wurster U, Dommasch U, Kappos L, Poser S. Cyclosporin A versus azathioprine in multiple sclerosis: seven year follow-up and long-term side effects. Journal of Neurology, Neurosurgery and Psychiatry 1994;57:516. [CENTRAL: CN-00225238]
Kappos 1990 {published data only}
Massacesi 2014 {published data only}
Milanese 1993 {published data only}
Milanese 2001 {published data only}
Putzki 2006 {published data only}
References to studies excluded from this review
Braun Hashemi 2006 {published data only}
-
- Braun Hashemi CA, Zang YC, Arbona JA, Bauerle JA, Frazer ML, Lee H, et al. Serum immunologic markers in multiple sclerosis patients on continuous combined therapy with beta-interferon 1a, prednisone and azathioprine. Multiple Sclerosis 2006;12(5):652-8. [DOI: 10.1177/1352458506070665] - DOI - PubMed
Caputo 1987 {published data only}
Cavazzuti 1997 {published data only}
Cendrowski 1971 {published data only}
Ellison 1984 {published data only}
Lhermitte 1984 {published data only}
Markovic‐Plese 2003 {published data only}
-
- Markovic-Plese S, Bielekova B, Kadom N, Leist TP, Martin R, Frank JA, et al. Longitudinal MRI study: the effects of azathioprine in MS patients refractory to interferon beta-1b. Neurology 2003;60(11):1849-51. [DOI: 10.1212/01.wnl.0000071218.34009.af] [PMID: ] - DOI - PubMed
Mertin 1982 {published data only}
Patzold 1978 {published data only}
-
- Patzold U, Haller P, Haas J, Pocklington P, Deicher H. Treatment of multiple sclerosis with levamisole and azathioprine. Comparison of the effectiveness, of an 'immunostimulative' and 'immunosuppressive' treatment (author's translation) [Therapie der Multiplen Sklerose mit Levamisol und Azathioprin. Vergleich der Wirksamkeit einer "immunstimulierenden" und "immunsuppressiven" Behandlung]. Nervenarzt 1978;49(5):285-94. [PMID: ] - PubMed
Patzold 1982 {published data only}
Ravnborg 2009 {published data only}
-
- Ravnborg M, Bendtzen K, Christensen O, Jensen PE, Hesse D, Tovey MG, et al. Treatment with azathioprine and cyclic methylprednisolone has little or no effect on bioactivity in anti-interferon beta antibody-positive patients with multiple sclerosis. Multiple Sclerosis 2009;15(3):323-8. [DOI: 10.1177/1352458508099476] - DOI - PubMed
Ring 1974 {published data only}
Rosen 1979 {published data only}
Steck 1990 {published data only}
Zeeberg 1985 {published data only}
-
- Zeeberg IE, Heltberg A, Fog T. Follow-up evaluation after at least two years' treatment with azathioprine in a double-blind trial. European Neurology 1985;24:435-6. [DOI: 10.1159/000115838] - DOI
Zeeberg 1986 {published data only}
-
- Zeeberg IE. Azathioprine assessment in progressive multiple sclerosis. Clinical aspects. In: Hommes OR, editors(s). Multiple Sclerosis Research in Europe. Lancaster, England: MTP Press, 1986:62-70. [DOI: 10.1007/978-94-009-4143-4_9] - DOI
References to studies awaiting assessment
Aimard 1978 {published data only}
-
- Aimard G, Confavreaux C, Trouillas P, Devic M. Treatment of multiple sclerosis by azathioprine. About 77 cases studied during 10 years (author's translation)] [L'azathioprine dans le traitement de la sclérose en plaques. Une expérience de 10 ans à propos de 77 malades]. Revue Neurologique 1978;134(3):215‐22. [PMID: ] - PubMed
Ciesielski 1974 {published data only}
-
- Ciesielski T, Burczyk-Popko I, Goralski H, Sypniewski J, Cendrowski W. Treatment of multiple sclerosis with azathioprine (Imuran) [Wyniki leczenie azatiopryna (imuranem) chorych na stwardnienie rozsiane]. Polski Tygodnik Lekarski 1974;29(3):101‐2. [PMID: ] - PubMed
Confavreux 1980 {published data only}
-
- Confavreux C, Aimard G, Devic M. Computerized analysis of 349 multiple sclerosis cases. Natural history of the disease. Evaluation of a continuous immunosuppressive therapeutic trial with azathioprine. Lyon Medical 1980;243(10):595-602. [EMBASE: L10061229]
Danielczyk 1973 {published data only}
-
- Danielczyk W. D penicillamine in the treatment of multiple sclerosis. Therapiewoche 1973;23(48):4704-10. [EMBASE: L4120570]
Ellison 1981 {published data only}
-
- Ellison GW, Myers LW, Shih WH. Immunologic aspects of azathioprine and steroid treatment of multiple sclerosis. Neurology 1981;31(4):147. [EMBASE: L11065456]
Frick 1971 {published data only}
-
- Frick E, Angstwurm H, Späth G. [Immunosuppressive therapy of multiple sclerosis. 1. Preliminary communication on the results of treatment with azathioprine and anti-lymphocytic globulin]. Munchener Medizinische Wochenschrift 1971;113(7):221-31. [PMID: ] - PubMed
Frick 1974a {published data only}
-
- Frick E, Angstwurm H, Strauss G. Immunsuppressive treatment of multiple sclerosis. 2. Critical review of general results (author's translation) [Immunsuppressive therapie der multiplen sklerose. 2. Kritischer bericht über allgemeine erfahrungen]. Munchener Medizinische Wochenschrift 1974;116(45):1987. [PMID: ] - PubMed
Frick 1974b {published data only}
-
- Frick E, Angstwurm H, Strauss G. Immunosuppressive treatment of multiple sclerosis. 3. Results of treatment with azathioprine and antilymphocytic globulin (author's translation) [Immunosuppressive therapie der multiplen sklerose. 3. Eigene behandlungsergebnisse mit azathioprin und antilymphozytenglobulin]. Munchener Medizinische Wochenschrift 1974;116(48):2105-12. [PMID: ] - PubMed
Frick 1977 {published data only}
-
- Frick E, Angstwurm H, Blomer R, Strauss G. Immunosuppressive treatment of multiple sclerosis. 4. Results of treatment with azathioprine and antilymphocyte globulin (author's translation) [Immunsuppressive therapie der multiplen sklerose. 4. Mitteilung: behandlungsergebnisse mit azathioprin und antilymphozytgenglobulin]. Munchener Medizinische Wochenschrift 1977;119(35):1111-4. [PMID: ] - PubMed
Gentile 1972 {published data only}
-
- Gentile A, Stella L. Preliminary clinical experiences obtained with immunological therapy of multiple sclerosis [Esperienze cliniche preliminari ottenute con una terapia "immunologica" nella sclerosi multipla]. Annali Sclavo 1972;14(6):726-32. [PMID: ] - PubMed
Ghezzi 1989 {published data only}
-
- Ghezzi A, Di Falco M, Locatelli C, Zaffaroni M, Caputo D, Marforio S, et al. Clinical controlled randomized trial of azathioprine in multiple sclerosis. In: Gonsette RE, Delmotte P, editors(s). Recent Advances in Multiple Sclerosis Therapy. Amsterdam: Elsevier, 1989:345–6. [CENTRAL: CN-00716237]
Göpel 1972 {published data only}
-
- Göpel W, Benkenstein H, Banzhaf M. [Immunosuppressive therapy of multiple sclerosis using cyclophosphamide and imuran. Report on 57 cases]. Das Deutsche Gesundheitswesen 1972;27(41):1955-61. [PMID: ] - PubMed
Handouk 2009 {published data only}
-
- Handouk Y, De Riso S, Perticaroli E, Danni M, Angeleri V, Provinciali L. Cancer risk in a MS population treated with immunosuppressants. Multiple Sclerosis 2009;15(9):S250. [EMBASE: L70100979]
Hervet 1974 {published data only}
-
- Hervet E, Barrat J, Darbois Y, Faguer C. Letter: Teratogenic effects of medications [Lettre: Effets tératogènes des médicaments]. Nouvelle Presse Medicale 1974;3(37):2419. [PMID: ] - PubMed
Hitzchke 1979 {published data only}
-
- Hitzchke B, Schumm N, Meyer-Rienecker H, Schroeter P. Efficacy and adverse effects of immunosuppressive therapy in diseases of the encephalomyelitis disseminata type (multiple sclerosis). Zentralblatt fur Allgemeine Pathologie und Pathologische Anatomie 1979;123(1):148-9. [EMBASE: L9213845]
Lhermitte 1984 {published data only}
-
- Lhermitte F, Marteau R, Roullet E, Saxcé H, Loridan M. [Prolonged treatment of multiple sclerosis with average doses of azathioprine. An evaluation of 15 years' experience]. Revue Neurologique 1984;140(10):553-8. [PMID: ] - PubMed
Schluep 1991 {published data only}
-
- Schluep M, Steck AJ, Despland PA, Regli F, Ochsner F, Berrut E, et al. Efficacy and tolerance of cyclosporin A in the treatment of multiple sclerosis [Efficacité et tolérance de la cyclosporine A dans le traitement de la sclérose en plaques]. Schweizerische Rundschau fur Medizin Praxis. 1991;80(24):670‐2. [PMID: ] - PubMed
Wilkerson 1975 {published data only}
-
- Wilkerson LD, Lisak RP, Zweiman B. Azathioprine therapy effects on antimyelin and other antibodies in multiple sclerosis. Federation Proceedings 1975;34(3):no. 4245. [EMBASE: L6027434]
Yankov 1980 {published data only}
-
- Yankov Y, Shotekov P. Combined immunosuppressive treatment of multiple sclerosis with azathioprine and prednisolone F. Vatrechni Bolesti 1980;19(2):135-40. [EMBASE: L11229718]
References to ongoing studies
EUDRACT 2006‐004937‐13 {published data only}
-
- EUDRACT 2006-004937-13. M.A.I.N. trial [Multicentee [sic] randomized controlled study of azathioprine versus iterferon beta in relapsing remitting multiple sclerosis]. www.clinicaltrialsregister.eu/ctr-search/trial/2006-004937-13/IT (first received 4 July 2007).
NCT03653273 {published data only}
-
- NCT03653273. Disease modifying therapies withdrawal in inactive secondary progressive multiple sclerosis patients older than 50 years (STOP-I-SEP). clinicaltrials.gov/ct2/show/NCT03653273 (first received 31 August 2018).
NCT04106830 {published data only}
-
- NCT04106830. Clinical and imaging patterns of neuroinflammation diseases in China (CLUE) [Prospective cohort study of clinical and imaging patterns of neuroinflammation diseases (CLUE)]. clinicaltrials.gov/ct2/show/NCT04106830 (first received 27 September 2019).
Additional references
AIFA 2021
-
- Drugs with well-established use in the treatment of neurological disorders for indications other than those covered by the marketing authorisation order [Farmaci con uso consolidato nel trattamento di patologie neurologiche per indicazioni anche differenti da quelle previste dal provvedimento di autorizzazione all’immissione in commercio]. www.aifa.gov.it/documents/20142/1288746/Allegato-4_Neurologia_04.01.2021... (accessed 15 January 2021).
Alonso 2008
Amato 1993
Benedict 2020
Brown 2019
Campbell 2020
Cannon 2020
Compston 2002
Confavreux 2000
Deeks 2020
-
- Deeks JJ, Higgins JP, Altman DG, editor(s). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (updated September 2020). Cochrane, 2020. Available from www.training.cochrane.org/handbook.
DerSimonian 1986
Elion 1993
EMA 2015
-
- Guideline on clinical investigation of medicinal products for the treatment of multiple sclerosis; 26 March 2015. www.ema.europa.eu/en/documents/scientific-guideline/guideline-clinical-i... (accessed 15 January 2021).
Filippini 2013
-
- Filippini G, Del Giovane C, Vacchi L, D’Amico R, Di Pietrantonj C, Beecher D, et al. Immunomodulators and immunosuppressants for multiple sclerosis: a network meta-analysis. Cochrane Database of Systematic Reviews 2013, Issue 6. Art. No: CD008933. [DOI: 10.1002/14651858.CD008933.pub2] - DOI - PMC - PubMed
Filippini 2017
-
- Filippini G, Del Giovane C, Clerico M, Beiki O, Mattoscio M, Piazza F, et al. Treatment with disease-modifying drugs for people with a first clinical attack suggestive of multiple sclerosis. Cochrane Database of Systematic Reviews 2017, Issue 4. Art. No: CD012200. [DOI: 10.1002/14651858.CD012200.pub2] - DOI - PMC - PubMed
GBD 2019
Ghezzi 2018
GRADEproGDT [Computer program]
-
- GRADEpro GDT. Version accessed 3 February 2023. Hamilton (ON): McMaster University (developed by Evidence Prime), 2023. Available at gradepro.org.
Greenflield 2018
Higgins 2003
Higgins 2017
-
- Higgins JP, Altman DG, Sterne JA, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s), Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). Cochrane, 2017. Available from training.cochrane.org/handbook.
Higgins 2020
-
- Higgins JP, Li T, Deeks JJ, editor(s). Chapter 6: Choosing effect measures and computing estimates of effect. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (updated September 2020). Cochrane, 2020. Available from www.training.cochrane.org/handbook.
Hommes 2004
Huskisson 1984
-
- Huskisson EC. Azathioprine. Clinical Rheumatology 1984;10(2):325-32. [PMID: ] - PubMed
Invernizzi 2008
Kieseier 2010
Kurtzke 1983
Lallana 2011
Langdon 2012
Laurson‐Doube 2021
Lee 2015
-
- Lee KM, Kim YS, Seo GS, Kim TO, Yang SK, IBD Study Group of the Korean Association for the Study of Intestinal Diseases. Use of thiopurines in inflammatory bowel disease: a Consensus Statement by the Korean Association for the Study of Intestinal Diseases (KASID). Intestinal Research 2015;13(3):193-207. [DOI: 10.5217/ir.2015.13.3.193] - DOI - PMC - PubMed
Lhermitte 1984
Lublin 1996
-
- Lublin FD, Reingold SC. Defining the clinical course of multiple sclerosis: results of an international survey. National Multiple Sclerosis Society (USA) Advisory Committee on clinical trials of new agents in multiple sclerosis. Neurology 1996;46(4):90-111. [DOI: 10.1212/wnl.46.4.907] - DOI - PubMed
Lublin 2014
Massacesi 2002
McAlpine 1968
-
- McAlpine D, Lumsden CE, Acheson ED. Multiple Sclerosis: A Reappraisal. Edinburgh: Livingstone, 1968. [ISBN: 0443008256]
McDonald 1977
-
- McDonald WI, Halliday AM. Diagnosis and classification of multiple sclerosis. British Medical Bulletin 1977;33:4-8. [PMID: ] - PubMed
McDonald 2001
McGuinness 2021
McKenzie 2020
-
- McKenzie JE, Brennan SE, Ryan RE, Thomson HJ, Johnston RV, Thomas J. Chapter 3: Defining the criteria for including studies and how they will be grouped for the synthesis. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (updated September 2020). Cochrane, 2020. Available from www.training.cochrane.org/handbook.
McWilliam 2020
Meyer‐Moock 2014
-
- Meyer-Moock S, Feng YS, Maeurer M, Dippel FW, Kohlmann T. Systematic literature review and validity evaluation of the Expanded Disability Status Scale (EDSS) and the Multiple Sclerosis Functional Composite (MSFC) in patients with multiple sclerosis. BMC Neurology 2014;14:58. [DOI: 10.1186/1471-2377-14-58] - DOI - PMC - PubMed
Microsoft Excel [Computer program]
-
- Microsoft Excel. Version 2405. Microsoft Corporation, 2024. Available from https://office.microsoft.com/excel.
Miller 2007
Na 2016
-
- Na R, Laaksonen MA, Grulich AE, Meagher NS, McCaughan GW, Keogh AM, et al. High azathioprine dose and lip cancer risk in liver, heart, and lung transplant recipients: a population-based cohort study. Journal of the American Academy of Dermatology 2016;74(6):1144–52. [DOI: 10.1016/j.jaad.2015.12.044] - DOI - PubMed
NCI 2021
-
- NCI's dictionary of cancer terms: short-term side effect. www.cancer.gov/publications/dictionaries/cancer-terms/def/short-term-sid... (accessed 15 January 2021).
Page 2021
Pasternak 2013
Paz 2015
Peryer 2023
-
- Peryer G, Golder S, Junqueira D, Vohra S, Loke YK. Chapter 19: Adverse effects. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.4 (updated August 2023). Cochrane, 2023. Available from training.cochrane.org/handbook.
Peters 2008
Poser 1983
Rae‐Grant 2018
Reeves 2020
-
- Reeves BC, Deeks JJ, Higgins JP, Shea B, Tugwell P, Wells GA. Chapter 24: Including non-randomized studies on intervention effects. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.1 (updated September 2020). Cochrane, 2020. Available from www.training.cochrane.org/handbook.
RevMan Web 2020 [Computer program]
-
- Review Manager Web (RevMan Web). Version 3.1.1. The Cochrane Collaboration, 2020. Available at revman.cochrane.org.
Ruggieri 2005
Rundles 1961
-
- Rundles RW, Laszlo J, Itoga T, Hobson JB, Garrison FE Jr. Clinical and hematologic study of 6[(1-methyl-4-nitro-5-imidazolyl)-thio] purine (B.W. 57-322) and related compounds. Cancer Chemotherapy Reports 1961;14:99-115. [PMID: ] - PubMed
Schumacher 1965
-
- Schumacher GA, Beebe G, Kibler RF, Kurland LT, Kurtzke JF, Mcdowell F, et al. Problems of experimental trials of therapy in multiple sclerosis: report by the panel on the evaluation of experimental trials of therapy in multiple sclerosis. Annals of the New York Academy of Sciences 1965;122:552-68. [DOI: 10.1111/j.1749-6632.1965.tb20235.x] [PMID: ] - DOI - PubMed
Sterne 2016
Tiede 2003
Tramacere 2015
-
- Tramacere I, Del Giovane C, Salanti G, D'Amico R, Filippini G. Immunomodulators and immunosuppressants for relapsing-remitting multiple sclerosis: a network meta-analysis. Cochrane Database of Systematic Reviews 2015, Issue 9. Art. No: CD011381. [DOI: 10.1002/14651858.CD011381.pub2] - DOI - PMC - PubMed
Tramacere 2023
-
- Tramacere I, Virgili G, Perduca V, Lucenteforte E, Benedetti MD, Capobussi M, et al. Adverse effects of immunotherapies for multiple sclerosis: a networkmeta-analysis. Cochrane Database of Systematic Reviews 2023, Issue 11. Art. No: CD012186. [DOI: 10.1002/14651858.CD012186.pub2] - DOI - PMC - PubMed
Trojano 2017
Vickrey 1995
Walton 2020
Weinshilboum 1980
Yamout 2019
-
- Yamout B, Sahraian M, Bohlega S, Al-Jumah M, Goueider R, Dahdaleh M, et al. Consensus recommendations for the diagnosis and treatment of multiple sclerosis: 2019 revisions to The MENACTRIMS Guidelines. Multiple Sclerosis and Related Disorders 2019;37:101459. [DOI: 10.1016/j.msard.2019.101459] - DOI - PubMed
Zeineddine 2020
References to other published versions of this review
Casetta 2007
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

