Acetate Shock Loads Enhance CO Uptake Rates of Anaerobic Microbiomes
- PMID: 39651844
- PMCID: PMC11626651
- DOI: 10.1111/1751-7915.70063
Acetate Shock Loads Enhance CO Uptake Rates of Anaerobic Microbiomes
Abstract
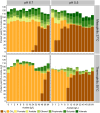
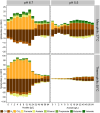
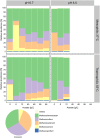
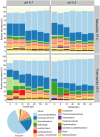
Pyrolysis of lignocellulosic biomass commonly produces syngas, a mixture of gases such as CO, CO2 and H2, as well as an aqueous solution generally rich in organic acids such as acetate. In this study, we evaluated the impact of increasing acetate shock loads during syngas co-fermentation with anaerobic microbiomes at different pH levels (6.7 and 5.5) and temperatures (37°C and 55°C) by assessing substrates consumption, metabolites production and microbial community composition. The anaerobic microbiomes revealed to be remarkably resilient and were capable of converting syngas even at high acetate concentrations of up to 64 g/L and pH 5.5. Modifying process parameters and acetate loads resulted in a shift of the product spectrum and microbiota composition. Specifically, a pH of 6.7 promoted methanogens such as Methanosarcina, whereas lowering the pH to 5.5 with lower acetate loads promoted the enrichment of syntrophic acetate oxidisers such as Syntrophaceticus, alongside hydrogenotrophic methanogens. Increasing acetate loads intensified the toxicity of undissociated acetic acid, thereby inhibiting methanogenic activity. Under non-methanogenic conditions, high acetate concentrations suppressed acetogenesis in favour of hydrogenogenesis and the production of various carboxylates, including valerate, with product profiles and production rates being contingent upon temperature. A possible candidate for valerate production was identified in Oscillibacter. Across all tested conditions, acetate supplementation provided additional carbon and energy to the mixed cultures and consistently increased carboxydotrophic conversion rates up to about 20-fold observed at pH 5.5, 55°C and 48 g/L acetate compared to control experiments. Species of Methanobacterium, Methanosarcina and Methanothermobacter may have been involved in CO biomethanation. Under non-methanogenic conditions, the bacterial species responsible for CO conversion remain unclear. These results offer promise for integrating process streams, such as syngas and wastewater, as substrates for mixed culture fermentation allowing for enhanced resource circularity, mitigation of environmental impacts and decreased dependence on fossil fuels.
Keywords: acetic acid; acetogenesis; anaerobic digestion; hydrogenogenesis; methanogenesis; open mixed cultures; syngas; syntrophic acetate oxidation.
© 2024 The Author(s). Microbial Biotechnology published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Asimakopoulos, K. , Łężyk M., Grimalt‐Alemany A., et al. 2019. “Temperature Effects on Syngas Biomethanation Performed in a Trickle Bed Reactor.” Chemical Engineering Journal 393, no. December: 124739. 10.1016/j.cej.2020.124739. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

