Safety, Pharmacokinetics, and Pharmacodynamics of a First-in-Class ClC-1 Inhibitor to Enhance Muscle Excitability: Phase I Randomized Controlled Trial
- PMID: 39651850
- PMCID: PMC11835425
- DOI: 10.1002/cpt.3516
Safety, Pharmacokinetics, and Pharmacodynamics of a First-in-Class ClC-1 Inhibitor to Enhance Muscle Excitability: Phase I Randomized Controlled Trial
Abstract
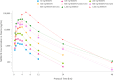
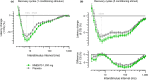
NMD670 is a first-in-class inhibitor of skeletal muscle-specific chloride channel ClC-1, developed to improve muscle weakness and fatigue in neuromuscular diseases. Preclinical studies show that ClC-1 inhibition enhances muscle excitability, improving muscle contractility and strength. We describe the first-in-human, randomized, double-blind, placebo-controlled study, which evaluated the safety, pharmacokinetics, and pharmacodynamics of single and multiple doses of NMD670 in healthy male and female subjects. Single-ascending doses (50-1,600 mg) were administered in a (partial) cross-over design; multiple-ascending doses (200-600 mg q.d.; 400 mg b.i.d.) were administered in a parallel design. Differences in pharmacokinetics between males/females and fed/fasted states were evaluated. Pharmacodynamic effects were evaluated using muscle velocity recovery cycles (MVRC) and analyzed using mixed-effects modeling. NMD670 was generally safe and well-tolerated in healthy subjects, with the only dose-related adverse event being myotonia occurring at the highest dose levels tested (single dose of 1,200, and 1,600 mg). Moreover, NMD670 significantly increased the following MVRC parameters after a single dose of 1,200 mg compared with placebo: early supernormality (estimated difference (ED) 2.04; 95% confidence interval (CI) 0.379, 3.70; P = 0.0242); early supernormality after 5 conditioning stimuli (ED 2.51; 95%CI 0.599, 4.41; P = 0.0177); supernormality at 20 ms (ED 2.78; 95%CI 1.377, 4.181; P = 0.0021). Importantly, the results of this study indicate pharmacological target engagement at well-tolerated dose levels in healthy subjects; firstly, because myotonia was an expected exaggerated on-target pharmacological effect, and secondly, because the effects on MVRC indicate increased muscle cell excitability. This study in healthy subjects indicates proof-of-mechanism and provides a solid base for translation to patients with neuromuscular diseases.
© 2024 The Author(s). Clinical Pharmacology & Therapeutics published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
This study was sponsored by NMD Pharma A/S. The funder had the following involvement with the study: study design, study oversight and medical monitoring, representation in dose escalation committee, decision to publish, and preparation of the manuscript. When this work was performed, J.H., J.B., T.S.G., K.G., E.C., J.Q., T.K.P., P.F., and T.H.P. were consultants or full‐time employees of NMD Pharma who may own and/or hold options/restricted stock units for the company. All other authors declared no competing interests for this work.
Figures


References
-
- Bækgaard Nielsen, O. , de Paoli, F.V. , Riisager, A. & Pedersen, T.H. Chloride channels take center stage in acute regulation of excitability in skeletal muscle: implications for fatigue. Physiology (Bethesda) 32, 425–434 (2017). - PubMed
-
- Gilhus, N.E. & Verschuuren, J.J. Myasthenia gravis: subgroup classification and therapeutic strategies. Lancet Neurol. 14, 1023–1036 (2015). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

