The gE/gI complex is necessary for kinesin-1 recruitment during alphaherpesvirus egress from neurons
- PMID: 39651860
- PMCID: PMC11784224
- DOI: 10.1128/jvi.01650-24
The gE/gI complex is necessary for kinesin-1 recruitment during alphaherpesvirus egress from neurons
Abstract
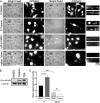
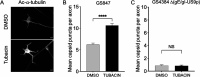
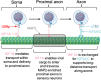
Following reactivation of a latent alphaherpesvirus infection, viral particles are assembled in neuronal cell bodies, trafficked anterogradely within axons to nerve termini, and spread to adjacent epithelial cells. The virally encoded membrane proteins US9p and the glycoprotein heterodimer gE/gI of pseudorabies virus (PRV) and herpes simplex virus type 1 (HSV-1) play critical roles in anterograde spread, likely as a tripartite gE/gI-US9p complex. Two kinesin motors, kinesin-1 and kinesin-3, are implicated in the egress of these viruses, but how gE/gI-US9p coordinates their activities is poorly understood. Here, we report that PRV, in addition to associating with the kinesin-3 motor KIF1A, recruits the neuronal kinesin-1 isoforms KIF5A and KIF5C, but not the broadly expressed isoform KIF5B, during egress from differentiated CAD neurons. Similarly, in the axons of dorsal root ganglia (DRG)-derived sensory neurons, PRV colocalized with KIF5C but not KIF5B. In differentiated CAD cells, the association of KIF1A with egressing PRV was dependent upon US9p, whereas the recruitment of KIF5 isoforms required gE/gI. Consistent with these findings, the number of PRV particles trafficking within CAD neurites and the axons of DRG neurons increased when kinesin-1 motor activity was upregulated by hyperacetylating microtubules using trichostatin A (TSA) or tubacin, and this enhanced trafficking depended upon the presence of gE/gI. We propose that, following its recruitment by US9p, KIF1A delivers PRV particles to a location where KIF5 motors are subsequently added by a gE/gI-dependent mechanism. KIF5A/C isoforms then serve to traffic viral particles along axons, resulting in characteristic recrudescent infection.
Importance: Alphaherpesviruses include important human and veterinary pathogens that share a unique propensity to establish life-long latent infections in the peripheral nervous system. Upon reactivation, these viruses navigate back to body surfaces and transmit to new hosts. In this study, we demonstrate that the virus gE/gI-US9p membrane complex routes virus particles down this complex neuronal egress pathway by coordinating their association with multiple kinesin microtubule motors.
Keywords: alphaherpesviruses; anterograde transport; axonal transport; herpes simplex virus; pseudorabies virus.
Conflict of interest statement
G.A.S. is a co-founder of Thyreos, Inc., and serves on the scientific advisory board of EG427. G.A.S. has stock ownership in both entities.
Figures
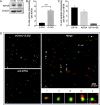

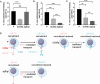
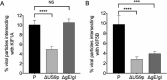

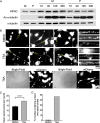
References
-
- Ambrosini AE, Borg KM, Deshmukh N, Berry MJ 2nd, Enquist LW, Hogue IB. 2024. Alpha herpesvirus exocytosis from neuron cell bodies uses constitutive secretory mechanisms, and egress and spread from axons is independent of neuronal firing activity. PLoS Pathog 20:e1012139. doi:10.1371/journal.ppat.1012139 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

