Physician Reasons for or Against Treatment Intensification in Patients With Metastatic Prostate Cancer
- PMID: 39652349
- PMCID: PMC11629128
- DOI: 10.1001/jamanetworkopen.2024.48707
Physician Reasons for or Against Treatment Intensification in Patients With Metastatic Prostate Cancer
Abstract
Importance: Clarifying the underutilization of treatment intensification (TI) for metastatic castration-sensitive prostate cancer (mCSPC) may improve implementation of evidence-based medicine and survival outcomes.
Objective: To investigate physicians' beliefs about TI in mCSPC to understand the gap between evidence-based guidelines and clinical practice.
Design, setting, and participants: This survey study analyzed data from the Adelphi Real World retrospective survey, which comprised physician surveys that were linked to medical record reviews of US adult patients treated for mCSPC between July 2018 and January 2022.
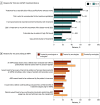
Main outcomes and measures: The survey included questions on physician and practice demographics. Physicians completed patient record forms, based on patient medical records with information including patient demographics, clinical characteristics, and patient management. Physicians recalled reasons for prescribing decisions using 48 precoded and open-text responses. Bivariate and multivariable analyses assessed the likelihood of their patients receiving first-line TI; the main outcome was the likelihood of their patients receiving TI using odds ratios (ORs).
Results: In total, 617 male patients met the analysis criteria (mean [SD] age, 68.6 [8.1] years). Among these patients, 349 (56.6%) were Medicare beneficiaries. Overall, 430 (69.7%) did not receive first-line TI with androgen receptor pathway inhibitors and/or chemotherapy. The 107 US-based physicians' top reasons for treatment choice for their patients were tolerability concerns (TI: 121 [64.7%]; no TI: 252 [58.6%]; P = .18) and following guideline recommendations (TI: 115 [61.5%]; no TI: 230 [53.5%]; P = .08). In the bivariate analysis, physicians seeking to reduce prostate-specific antigen (PSA) by 75% to 100% were more likely to provide first-line TI compared with physicians who aimed to lower PSA by 0% to 49% (OR, 1.63 [95% CI, 1.04-2.56]; P = .03). In the multivariable analysis, patients whose physicians based treatment choice on guidelines were more likely to receive TI than patients whose physicians did not report this reason (OR, 3.46 [95% CI, 1.32-9.08]; P = .01).
Conclusions and relevance: The findings of this study, which analyzed data from a medical records-linked clinical practice survey, indicated low rates of first-line TI for mCSPC despite guideline recommendations. Barriers to TI included lack of knowledge about guidelines and published efficacy and safety data. Physicians with greater PSA reduction goals were more likely to use TI. Physician education on treatment guidelines and clinical trial data, while raising expectations for PSA response, may increase rates of first-line TI in mCSPC.
Conflict of interest statement
Figures

Comment in
References
-
- James ND, Sydes MR, Clarke NW, et al. ; STAMPEDE Investigators . Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016;387(10024):1163-1177. doi: 10.1016/S0140-6736(15)01037-5 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

