Care Models to Improve Pain and Reduce Opioids Among Patients Prescribed Long-Term Opioid Therapy: The VOICE Randomized Clinical Trial
- PMID: 39652356
- PMCID: PMC11791716
- DOI: 10.1001/jamainternmed.2024.6683
Care Models to Improve Pain and Reduce Opioids Among Patients Prescribed Long-Term Opioid Therapy: The VOICE Randomized Clinical Trial
Abstract
Importance: Patients prescribed long-term opioid therapy for chronic pain often experience unrelieved pain, poor quality of life, and serious adverse events.
Objective: To compare the effects of integrated pain team (IPT) vs pharmacist collaborative management (PCM) on pain and opioid dosage.
Design, setting, and participants: This study was a pragmatic multisite 12-month randomized comparative effectiveness trial with masked outcome assessment. Patients were recruited from October 2017 to March 2021; follow-up was completed June 2022. The study sites were Veterans Affairs primary care clinics. Eligible patients had moderate to severe chronic pain despite long-term opioid therapy (≥20 mg/d for at least 3 months).
Interventions: IPT involved interdisciplinary pain care planning, visits throughout 12 months with medical and mental health clinicians, and emphasis on nondrug therapies and motivational interviewing. PCM was a collaborative care intervention involving visits throughout 12 months with a clinical pharmacist care manager who conducted structured monitoring and medication optimization. Both interventions provided individualized pain care and opioid tapering recommendations to patients.
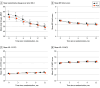
Main outcomes and measures: The primary outcome was pain response (≥30% decrease in Brief Pain Inventory total score) at 12 months. The main secondary outcome was 50% or greater reduction in opioid daily dosage at 12 months.
Results: A total of 820 patients were randomized to IPT (n = 411) or PCM (n = 409). Participants' mean (SD) age was 62.2 (10.6) years, and 709 (86.5%) were male. A pain response was achieved in 58/350 patients in the IPT group (16.4%) vs 54/362 patients in the PCM group (14.9%) (odds ratio, 1.11 [95% CI, 0.74-1.67]; P = .61). A 50% opioid dose reduction was achieved in 102/403 patients in the IPT group (25.3%) vs 98/399 patients in the PCM group (24.6%) (odds ratio, 1.03 [95% CI, 0.75-1.42]; P = .85). Over 12 months, the mean (SD) Brief Pain Inventory total score improved from 6.7 (1.5) points to 6.1 (1.8) points (P < .001) in IPT and from 6.6 (1.6) points to 6.0 (1.9) points (P < .001) in PCM (between-group P = .82). Over 12 months, mean (SD) opioid daily dosage decreased from 80.8 (74.2) mg/d to 54.2 (65.0) mg/d in IPT (P < .001) and from 74.5 (56.9) mg/d to 52.8 (51.9) mg/d (P < .001) in PCM (between-group P = .22).
Conclusions and relevance: Outcomes in this randomized clinical trial did not differ between groups; both had small improvements in pain and substantial reductions in opioid dosage.
Trial registration: ClinicalTrials.gov Identifier: NCT03026790.
Conflict of interest statement
Figures
References
-
- Dahlhamer JM, Connor EM, Bose J, Lucas JL, Zelaya CE. Prescription opioid use among adults with chronic pain: United States, 2019. Natl Health Stat Report. 2021;(162):1-9. - PubMed
-
- Eriksen J, Sjøgren P, Bruera E, Ekholm O, Rasmussen NK. Critical issues on opioids in chronic non-cancer pain: an epidemiological study. Pain. 2006;125(1-2):172-179. - PubMed
-
- Sjøgren P, Grønbæk M, Peuckmann V, Ekholm O. A population-based cohort study on chronic pain: the role of opioids. Clin J Pain. 2010;26(9):763-769. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

