An endogenous aryl hydrocarbon receptor ligand induces preeclampsia-like phenotypes in rats
- PMID: 39652430
- PMCID: PMC11737537
- DOI: 10.1113/JP287503
An endogenous aryl hydrocarbon receptor ligand induces preeclampsia-like phenotypes in rats
Abstract
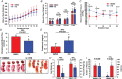
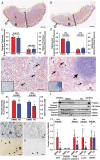
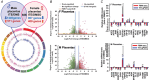
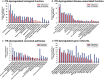
Preeclampsia (PE) is a hypertensive disorder during human pregnancy. Aryl hydrocarbon receptor (AhR) is a ligand-activated transcription factor. Exogenous and endogenous AhR ligands can induce hypertension in male rats and mice. Herein, using rats as a model, we tested the hypothesis that over-regulation of endogenous AhR ligands during pregnancy impairs vascular functions by disrupting the transcriptome in the placenta, contributing to the development of PE. Pregnant rats were injected daily with an endogenous AhR ligand, 2-(1'H-indole-3'-carbonyl)-thiazole-4-carboxylic acid methyl ester (ITE), from gestational day (GD) 10 to 19. Maternal mean blood pressure was measured on GD16-20. Proteinuria and uteroplacental blood flow were monitored on GD20. Placentas collected on GD20 were used to determine changes in vascular density and transcriptome. Compared with the vehicle control, ITE elevated maternal mean blood pressure by 22% and 16% on GD16 and 17, respectively. ITE increased proteinuria by 50% and decreased uteroplacental blood flow by 26%. ITE reduced the placental vascular density by 18%. RNA sequencing analysis revealed that ITE induced 1316 and 2020 differentially expressed genes (DEGs) in female and male placentas, respectively. These DEGs were enriched in pathways relevant to heart diseases, vascular functions and inflammation. Bioinformatics analysis also predicted that ITE altered immune cell infiltration in placentas depending on fetal sex. These data suggest that over-regulation of endogenous AhR ligands may lead to PE with impaired vascular functions and disrupted fetal sex-specific transcriptomes and immune cell infiltration in placentas. These AhR ligand-induced DEGs and pathways may represent promising therapeutic targets for PE-induced cardiovascular dysfunctions. KEY POINTS: An endogenous AhR ligand (ITE) elevated maternal blood pressure and proteinuria in pregnant rats, and decreased uteroplacental blood flow and fetal and placental growth, all of which are hallmarks of preeclampsia. ITE reduced vascular density and altered immune cell distribution in rat placentas. ITE dysregulated transcriptomes in rat placentas in a fetal sex-specific manner. These ITE-dysregulated genes and pathways are highly relevant to diseases of heart, vascular functions and inflammatory responses.
Keywords: AhR ligand; placentas; preeclampsia; sexual dimorphism; transcriptomics; vasculature.
© 2024 The Author(s). The Journal of Physiology published by John Wiley & Sons Ltd on behalf of The Physiological Society.
Conflict of interest statement
The authors have no conflict of interest.
Figures
Update of
-
An Endogenous Aryl Hydrocarbon Receptor Ligand Induces Preeclampsia-like Phenotypes: Transcriptome, Phosphoproteome, and Cell Functions.bioRxiv [Preprint]. 2023 Dec 26:2023.12.20.572271. doi: 10.1101/2023.12.20.572271. bioRxiv. 2023. Update in: J Physiol. 2025 Jan;603(2):579-594. doi: 10.1113/JP287503. PMID: 38187714 Free PMC article. Updated. Preprint.
References
-
- Burton, G. J. , Reshetnikova, O. S. , Milovanov, A. P. , & Teleshova, O. V. (1996). Stereological evaluation of vascular adaptations in human placental villi to differing forms of hypoxic stress. Placenta, 17(1), 49–55. - PubMed
-
- Chang, C. C. , Hsu, Y. H. , Chou, H. C. , Lee, Y. G. , & Juan, S. H. (2017). 3‐methylcholanthrene/Aryl‐hydrocarbon receptor‐mediated hypertension through eNOS inactivation. Journal of Cellular Physiology, 232(5), 1020–1029. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

