Imlunestrant Is an Oral, Brain-Penetrant Selective Estrogen Receptor Degrader with Potent Antitumor Activity in ESR1 Wild-Type and Mutant Breast Cancer
- PMID: 39652577
- PMCID: PMC11831106
- DOI: 10.1158/0008-5472.CAN-24-2608
Imlunestrant Is an Oral, Brain-Penetrant Selective Estrogen Receptor Degrader with Potent Antitumor Activity in ESR1 Wild-Type and Mutant Breast Cancer
Abstract
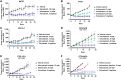
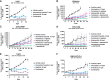
Targeting of the estrogen receptor (ER) by antiestrogens is the standard of care for patients with ER+ HER2- advanced/metastatic breast cancer. Although antiestrogens that degrade ERα (fulvestrant) or block estrogen production (aromatase inhibitors) have improved patient outcomes, clinically important challenges remain related to drug administration, limited bioavailability, lack of brain exposure, and acquired resistance due to ESR1 mutations. These limitations indicate a need for more robust ER-targeted therapies. Here, we discovered and characterized imlunestrant, a next-generation potent, brain-penetrant oral selective ER degrader. Imlunestrant degraded ERα and decreased ERα-mediated gene expression both in vitro and in vivo. Cell proliferation and tumor growth in ESR1 wild-type (WT) and mutant models were significantly inhibited by imlunestrant. Combining imlunestrant with abemaciclib (CDK4/6 inhibitor), alpelisib (PI3K inhibitor), or everolimus (mTOR inhibitor) further enhanced tumor growth inhibition, regardless of ESR1 mutational status. In an ER+ breast cancer intracranial tumor model, imlunestrant prolonged survival compared with vehicle or alternative selective ER degrader therapies. Together, these findings support the potential of imlunestrant to degrade ERα and suppress the growth of ESR1-WT and mutant breast cancer, including brain metastatic tumors. Significance: Imlunestrant, a next-generation, brain-penetrant oral ERα degrader, displays potent activity in ESR1 wild-type and mutant breast cancer, enhances combination activity with standard-of-care agents, and inhibits growth of ER+ intracranial tumors.
©2024 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
S.V. Bhagwat reports other support from Eli Lilly and Company outside the submitted work, as well as a patent for US20220288019 pending. M. Vandekopple reports other support from Eli Lilly and Company outside the submitted work, as well as a patent for PCT/US2024/027427 pending. W. Shen reports other support from Eli Lilly and Company outside the submitted work. A. Capen reports a patent for PCT/US2024/027427 pending. L. Huber reports a patent filed pending. M.A. Castanares reports a patent for PCT/US2024/027427 pending. J.D. Cohen reports a patent for US10654866 issued and a patent for US12128040 issued. J. Bastian reports a patent for US10654866 issued. E. Yuen reports a patent for US17/689286 pending. V. Rodriguez Cruz reports a patent for PCT/US2024/027427 pending. N. Pulliam reports other support from Eli Lilly and Company during the conduct of the study. M.S. Dowless reports personal fees from Eli Lilly and Company outside the submitted work, as well as a patent for PCT/US2024/027427 pending. L. Puca reports a patent for PCT/US2024/027427 pending. A. Klippel reports a patent for PCT/US2024/027427 pending. F. Bacchion reports other support from Eli Lilly and Company outside the submitted work. V. Rodrik-Outmezguine reports other support from Eli Lilly and Company outside the submitted work. X. Gong reports other support from Eli Lilly and Company outside the submitted work, as well as a patent filed pending. No disclosures were reported by the other authors.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

