Mosquito-independent milk-associated transmission of zoonotic Wesselsbron virus in sheep
- PMID: 39652585
- PMCID: PMC11658706
- DOI: 10.1371/journal.ppat.1012751
Mosquito-independent milk-associated transmission of zoonotic Wesselsbron virus in sheep
Abstract
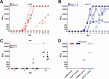
Wesselsbron virus (WSLV) is a zoonotic, mosquito-borne orthoflavivirus endemic to sub-Saharan Africa, causing abortions and stillbirths in small ruminants. The life cycle of WSLV involves Aedes mosquitoes and various wildlife and domestic animals. Seminal studies in the 1950s have shown the zoonotic potential of WSLV, notably in accidental infections of laboratory workers exposed to infected material. More recent epidemiological studies suggest the emergence of clade I WSLV strains in peri-domestic and rural areas of western and eastern Africa. The pathobiology of recent clade I WSLV strains is unknown and no virus isolate is available. To address these gaps, we generated a recombinant clade I WSLV SA999 infectious clone (rSA999) by reverse genetics. Subsequently, lactating ewes were inoculated intravenously with the WSLV rSA999 strain or the clade II SAH177 strain in insect-free biocontainment stables. Inoculated ewes developed fever, viremia, and showed high levels of viral RNA at mucosal surfaces, and elevated viral titers in milk. Milk production was reduced, which directly affected the growth of the lambs, particularly within the rSA999 group. The ewes with higher WSLV titers in their milk in each group transmitted the infection to their lambs, which developed fever, prolonged viremia, and virus secretion. All infected animals produced high antibody titers with cross-neutralizing activity against both WSLV strains. Histopathology and blood biochemistry analysis indicated liver damage associated with necrotizing hepatitis lesions and active viral replication in some cases, which was more pronounced in the rSA999 group. Notably, only the SAH177-infected animals exhibited lesions consistent with meningoencephalitis, suggesting that WSLV clade II strains are neurotropic and that clade I strain are more hepatotropic. These findings demonstrate a previously unrecognized mode of vector-free transmission of WSLV that raises significant concerns for public and animal health.
Copyright: © 2024 Zimoch et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Meurens F, Dunoyer C, Fourichon C, Gerdts V, Haddad N, Kortekaas J, et al. Animal board invited review: Risks of zoonotic disease emergence at the interface of wildlife and livestock systems. Animal. 2021;15(6):100241. Epub 20210603. doi: 10.1016/j.animal.2021.100241 ; PubMed Central PMCID: PMC8172357. - DOI - PMC - PubMed
-
- Walsh MG, Sawleshwarkar S, Hossain S, Mor SM. Whence the next pandemic? The intersecting global geography of the animal-human interface, poor health systems and air transit centrality reveals conduits for high-impact spillover. One Health. 2020;11:100177. Epub 20201008. doi: 10.1016/j.onehlt.2020.100177 ; PubMed Central PMCID: PMC7543735. - DOI - PMC - PubMed

