A potent and selective reaction hijacking inhibitor of Plasmodium falciparum tyrosine tRNA synthetase exhibits single dose oral efficacy in vivo
- PMID: 39652589
- PMCID: PMC11671014
- DOI: 10.1371/journal.ppat.1012429
A potent and selective reaction hijacking inhibitor of Plasmodium falciparum tyrosine tRNA synthetase exhibits single dose oral efficacy in vivo
Abstract
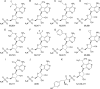
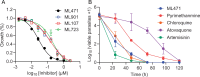
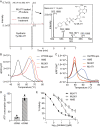
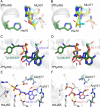
The Plasmodium falciparum cytoplasmic tyrosine tRNA synthetase (PfTyrRS) is an attractive drug target that is susceptible to reaction-hijacking by AMP-mimicking nucleoside sulfamates. We previously identified an exemplar pyrazolopyrimidine ribose sulfamate, ML901, as a potent reaction hijacking inhibitor of PfTyrRS. Here we examined the stage specificity of action of ML901, showing very good activity against the schizont stage, but lower trophozoite stage activity. We explored a series of ML901 analogues and identified ML471, which exhibits improved potency against trophozoites and enhanced selectivity against a human cell line. Additionally, it has no inhibitory activity against human ubiquitin-activating enzyme (UAE) in vitro. ML471 exhibits low nanomolar activity against asexual blood stage P. falciparum and potent activity against liver stage parasites, gametocytes and transmissible gametes. It is fast-acting and exhibits a long in vivo half-life. ML471 is well-tolerated and shows single dose oral efficacy in the SCID mouse model of P. falciparum malaria. We confirm that ML471 is a reaction hijacking inhibitor that is converted into a tight binding Tyr-ML471 conjugate by the PfTyrRS enzyme. A crystal structure of the PfTyrRS/ Tyr-ML471 complex offers insights into improved potency, while molecular docking into UAE provides a rationale for improved selectivity.
Copyright: © 2024 Xie et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
LM, SCH, YH, RG, DE, EdlC, SL are employees of Takeda Pharmaceuticals and owners of Takeda stock. The other authors have no competing interests to declare.
Figures


References
-
- World_Health_Organisation. WHO World Malaria Report 2023. Geneva: World Health Organization; Licence: CC BY-NC-SA 30 IGO. 2023.
-
- van der Pluijm RW, Imwong M, Chau NH, Hoa NT, Thuy-Nhien NT, Thanh NV, et al. Determinants of dihydroartemisinin-piperaquine treatment failure in Plasmodium falciparum malaria in Cambodia, Thailand, and Vietnam: a prospective clinical, pharmacological, and genetic study. Lancet Infect Dis. 2019;19(9):952–61. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

