Isatuximab, Lenalidomide, Bortezomib, and Dexamethasone Induction Therapy for Transplant-Eligible Newly Diagnosed Multiple Myeloma: Final Part 1 Analysis of the GMMG-HD7 Trial
- PMID: 39652594
- PMCID: PMC11974620
- DOI: 10.1200/JCO-24-02266
Isatuximab, Lenalidomide, Bortezomib, and Dexamethasone Induction Therapy for Transplant-Eligible Newly Diagnosed Multiple Myeloma: Final Part 1 Analysis of the GMMG-HD7 Trial
Abstract
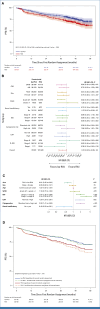
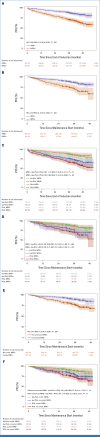
Previously, addition of isatuximab (Isa) to standard-of-care lenalidomide-bortezomib-dexamethasone (RVd) in transplant-eligible patients with newly diagnosed multiple myeloma in the GMMG-HD7 trial (ClinicalTrials.gov identifier: NCT03617731) resulted in a significant increase of minimal residual disease negativity (MRD-) rates after induction therapy. A total of 662 patients were randomly assigned to receive induction therapy with Isa-RVd (n = 331) or RVd (n = 329), followed by single or tandem autologous stem-cell transplant and second random assignment to maintenance with lenalidomide alone or Isa-lenalidomide. We report updated results for part 1 from first random assignment to post-transplant. As of January 31, 2024, MRD- rates continued to deepen after transplant (66% Isa-RVd v 48% RVd). Isa-RVd induction therapy significantly prolonged progression-free survival (PFS) compared with RVd regardless of maintenance therapy (hazard ratio, 0.70 [95% CI, 0.52 to 0.95]; P = .0184). Weighted risk set estimator analysis accounting for second random assignment followed by maintenance with only lenalidomide confirmed a statistically significant benefit for Isa-RVd followed by lenalidomide maintenance versus RVd followed by lenalidomide maintenance (stratified weighted log-rank test P = .016). In conclusion, after 18-week induction therapy followed by transplant without consolidation therapy, adding Isa to RVd resulted in a significant PFS benefit, regardless of maintenance strategy.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
No other potential conflicts of interest were reported.
Figures
References
-
- Channar A, Naqvi SAA, Khan MA, et al. : Quadruplet therapy for transplant-eligible newly diagnosed multiple myeloma (TENDMM): A systematic review and meta-analysis. J Clin Oncol 42, 2024. (suppl 16; abstr e19534)
-
- Facon T, Dimopoulos MA, Leleu XP, et al. : Isatuximab, bortezomib, lenalidomide, and dexamethasone for multiple myeloma. N Engl J Med 391:1597-1609, 2024 - PubMed
-
- US Food and Drug Administration: FDA approves Isatuximab-Irfc with bortezomib, lenalidomide, and dexamethasone for newly diagnosed multiple myeloma. 2024. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-appro...
-
- Goldschmidt H, Mai EK, Bertsch U, et al. : Addition of isatuximab to lenalidomide, bortezomib, and dexamethasone as induction therapy for newly diagnosed, transplantation-eligible patients with multiple myeloma (GMMG-HD7): Part 1 of an open-label, multicentre, randomised, active-controlled, phase 3 trial. Lancet Haematol 9:e810-e821, 2022 - PubMed
-
- Rajkumar SV, Dimopoulos MA, Palumbo A, et al. : International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol 15:e538-e548, 2014 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

