Association of Blood-Based Biomarkers and 6-Month Patient-Reported Outcomes in Patients With Mild TBI: A CENTER-TBI Analysis
- PMID: 39652812
- PMCID: PMC11675711
- DOI: 10.1212/WNL.0000000000210040
Association of Blood-Based Biomarkers and 6-Month Patient-Reported Outcomes in Patients With Mild TBI: A CENTER-TBI Analysis
Abstract
Background and objectives: There is seemingly contradictory evidence concerning relationships between day-of-injury biomarkers and outcomes after mild traumatic brain injury (mTBI). To address this issue, we examined the association between a panel of biomarkers and multidimensional TBI outcomes.
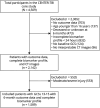
Methods: Participants with mTBI (Glasgow coma scores [GCSs] 13-15) were selected from Collaborative European NeuroTrauma Effectiveness Research in Traumatic Brain Injury, a European observational study recruiting patients with TBI with indication for brain CT and presentation within 24 hours. Exclusion criteria for this secondary analysis were age younger than 16 years, incomplete biomarker panel, death, or no recorded outcomes. Participants were separated into 2 groups, CT-negative and CT-positive. Multivariable binary logistic regression was used to assess the relation between the log biomarker level (glial fibrillary acidic protein [GFAP], neurofilament light [NfL], neuron-specific enolase [NSE], S100 calcium-binding protein B [S100B], tau, ubiquitin C-terminal hydrolase L1 [UCH-L1]) and dichotomized 6-month outcomes (functional outcomes [GOSE score <8], health-related quality of life [HRQoL; Quality of Life after Brain Injury-Overall Scale (QOLIBRI-OS) score <52, Short-Form 12-Item Survey version 2 Mental Component Summary (SF12v2 MCS) score <40, Short-Form 12-Item Survey version 2 Physical Component Summary (SF12v2 PCS) score <40], persistent postconcussion symptoms [Rivermead Post-Concussion Symptoms Questionnaire score ≥16], anxiety disorder [Generalized Anxiety Disorder-7 (GAD-7) score ≥8], depression [Patient Health Questionnaire-9 (PHQ-9) score ≥10], and post-traumatic stress disorder [PTSD Checklist for DSM-5 (PCL-5) score ≥33]).
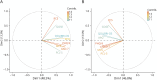
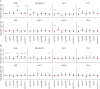
Results: A total of 1,589 participants (865 CT-negative, 724 CT-positive) were included (77% GCS 15, median age 52 years, 66% male). Higher biomarker levels were associated with a GOSE score <8: CT-negative: S100B (odds ratio [OR] 1.78, 95% CI 1.43-2.23) and UCH-L1 (OR 1.16, 95% CI 1.01-1.33); CT-positive: GFAP (OR 1.22, 95% CI 1.11-1.36), NfL (OR 1.30, 95% CI 1.11-1.52), S100B (OR 1.51, 95% CI 1.23-1.86), tau (OR 1.36, 95% CI 1.17-1.59), and UCH-L1 (OR 1.34, 95% CI 1.17-1.53). In CT-positive participants, positive association was seen between NfL (OR 1.3, 95% CI 1.06-1.60) and UCH-L1 (OR 1.28, 95% CI 1.07-1.54) with QOLIBRI-OS; S100B (OR 1.32, 95% CI 1.02-1.70) with SF12v2 PCS; and NSE (OR 1.52, 95% CI 1.06-2.18) and UCH-L1 (OR 1.21, 95% CI 1.01-1.46) with the GAD-7. However, in CT-negative participants only, negative associations were seen between GFAP and impairment on the QOLIBRI-OS (OR 0.76, 95% CI 0.66-0.88), SF12v2 MCS (OR 0.71, 95% CI 0.61-0.82), SF12v2 PCS (OR 0.79, 95% CI 0.68-0.91), GAD-7 (OR 0.80, 0.68-0.95), PHQ-9 (OR 0.80, 95% CI 0.68-0.93), and PCL-5 (OR 0.80, 95% CI 0.66-0.97).
Discussion: Participants with higher biomarker levels had greater odds of impaired functional recovery. However, in CT-negative participants, higher GFAP concentrations were associated with better HRQoL and less impaired mental health. Further exploration is required of the patient phenotypes that may explain the relationships observed in this analysis.
Conflict of interest statement
D.P. Whitehouse reports no disclosures relevant to the manuscript. L. Wilson reports receiving fees from Novartis and NeuroTrauma Sciences (USA) outside this study. E. Czeiter, A. Buki, K.K.W. Wang, N. von Steinbüchel, M. Zeldovich, and E. Steyerberg report no disclosures relevant to the manuscript. A.I.R. Maas reports receiving fees from Novartis, NeuroTrauma Sciences, and PressuraNeuro outside this study. D.K. Menon reports grants, personal fees, and nonfinancial support from GlaxoSmithKline Ltd.; grants, personal fees, and other from NeuroTrauma Sciences; grants and personal fees from Integra Life Sciences; personal fees from Pfizer Ltd.; grants and personal fees from Lantmannen AB; from Calico Ltd.; personal fees from Pressura Neuro Ltd.; and others from Cortirio Ltd., outside the submitted work. V.F.J. Newcombe holds a grant with ROCHE Pharmaceuticals, outside the submitted work. Go to
Figures

References
-
- Nelson LD, Temkin NR, Dikmen S, et al. Recovery after mild traumatic brain injury in patients presenting to US level I trauma centers: a transforming research and clinical knowledge in traumatic brain injury (TRACK-TBI) study. JAMA Neurol. 2019;76(9):1049-1059. doi: 10.1001/jamaneurol.2019.1313 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
