Biomarker-Guided Antibiotic Duration for Hospitalized Patients With Suspected Sepsis: The ADAPT-Sepsis Randomized Clinical Trial
- PMID: 39652885
- PMCID: PMC11862976
- DOI: 10.1001/jama.2024.26458
Biomarker-Guided Antibiotic Duration for Hospitalized Patients With Suspected Sepsis: The ADAPT-Sepsis Randomized Clinical Trial
Erratum in
-
Clarification of Noninferiority Wording and Corrected eFigure.JAMA. 2026 Jan 13;335(2):187. doi: 10.1001/jama.2025.25495. JAMA. 2026. PMID: 41405878 Free PMC article. No abstract available.
Abstract
Importance: For hospitalized critically ill adults with suspected sepsis, procalcitonin (PCT) and C-reactive protein (CRP) monitoring protocols can guide the duration of antibiotic therapy, but the evidence of the effect and safety of these protocols remains uncertain.
Objective: To determine whether decisions based on assessment of CRP or PCT safely results in a reduction in the duration of antibiotic therapy.
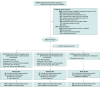
Design, setting, and participants: A multicenter, intervention-concealed randomized clinical trial, involving 2760 adults (≥18 years), in 41 UK National Health Service (NHS) intensive care units, requiring critical care within 24 hours of initiating intravenous antibiotics for suspected sepsis and likely to continue antibiotics for at least 72 hours.
Intervention: From January 1, 2018, to June 5, 2024, 918 patients were assigned to the daily PCT-guided protocol, 924 to the daily CRP-guided protocol, and 918 assigned to standard care.
Main outcomes and measures: The primary outcomes were total duration of antibiotics (effectiveness) and all-cause mortality (safety) to 28 days. Secondary outcomes included critical care unit data and hospital stay data. Ninety-day all-cause mortality was also collected.
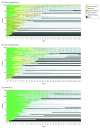
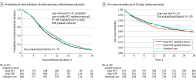
Results: Among the randomized patients (mean age 60.2 [SD, 15.4] years; 60.3% males), there was a significant reduction in antibiotic duration from randomization to 28 days for those in the daily PCT-guided protocol compared with standard care (mean duration, 10.7 [SD, 7.6] days for standard care and 9.8 [SD, 7.2] days for PCT; mean difference, 0.88 days; 95% CI, 0.19 to 1.58, P = .01). For all-cause mortality up to 28 days, the daily PCT-guided protocol was noninferior to standard care, where the noninferiority margin was set at 5.4% (19.4% [170 of 878] of patients receiving standard care; 20.9% [184 of 879], PCT; absolute difference, 1.57; 95% CI, -2.18 to 5.32; P = .02). No difference was found in antibiotic duration for standard care vs daily CRP-guided protocol (mean duration, 10.6 [7.7] days for CRP; mean difference, 0.09; 95% CI, -0.60 to 0.79; P = .79). For all-cause mortality, the daily CRP-guided protocol was inconclusive compared with standard care (21.1% [184 of 874] for CRP; absolute difference, 1.69; 95% CI, -2.07 to 5.45; P = .03).
Conclusions and relevance: Care guided by measurement of PCT reduces antibiotic duration safely compared with standard care, but CRP does not. All-cause mortality for CRP was inconclusive.
Trial registration: isrctn.org Identifier: ISRCTN47473244.
Conflict of interest statement
Figures
References
-
- Wirz Y, Meier MA, Bouadma L, et al. Effect of procalcitonin-guided antibiotic treatment on clinical outcomes in intensive care unit patients with infection and sepsis patients: a patient-level meta-analysis of randomized trials. Crit Care. 2018;22(1):191. doi: 10.1186/s13054-018-2125-7 - DOI - PMC - PubMed
-
- Westwood M, Ramaekers B, Whiting P, et al. Procalcitonin testing to guide antibiotic therapy for the treatment of sepsis in intensive care settings and for suspected bacterial infection in emergency department settings: a systematic review and cost-effectiveness analysis. Health Technol Assess. 2015;19(96):v-xxv, 1-236. doi: 10.3310/hta19960 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

