Cost-effectiveness of long-acting progestogens versus the combined oral contraceptives pill for preventing recurrence of endometriosis-related pain following surgery: an economic evaluation alongside the PRE-EMPT trial
- PMID: 39653562
- PMCID: PMC11628948
- DOI: 10.1136/bmjopen-2024-088072
Cost-effectiveness of long-acting progestogens versus the combined oral contraceptives pill for preventing recurrence of endometriosis-related pain following surgery: an economic evaluation alongside the PRE-EMPT trial
Abstract
Objectives: To evaluate the cost-effectiveness of long-acting progestogens (LAP), including levonorgestrel-releasing intrauterine system (LNG-IUS) and depot-medroxyprogesterone acetate (DMPA), compared with the combined oral contraceptives pill (COCP) in preventing recurrence of endometriosis-related pain postsurgery.
Design: Within-trial economic evaluation alongside a multicentre, pragmatic, parallel-group, open-label, randomised controlled trial (Preventing Recurrence of Endometriosis by means of Long-Acting Progestogen Therapy trial).
Setting: Thirty-four UK hospitals recruiting participants from November 2015 to March 2019.
Patients: Four hundred and five women aged 16-45 years undergoing conservative endometriosis surgery.
Interventions: The ratio of 1:1 randomisation to receive LAPs (LNG-IUS or DMPA) or COCP.
Main outcome measures: The primary evaluation was a cost-utility analysis based on cost per quality-adjusted life-year (QALY) gained at 3 years. We adopted a UK National Health Service perspective. Secondary analyses in the form of cost-effectiveness analysis based on a range of outcomes were also undertaken.
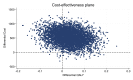
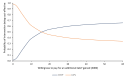
Results: For the primary analysis, the COCP group incurred an additional cost of £533 (95% CI £52 to £983) per woman compared with LAPs. Treatment with COCP generated additional QALYs of 0.031 (95% CI -0.079 to 0.139) compared with the LAP group over 36-month follow-up. The incremental cost-effectiveness ratio for COCP compared with LAPs is therefore approximately £17 193 per QALY. The probabilistic sensitivity analysis suggested that there was a 54.7% probability that COCP would be cost-effective at the £20 000/QALY threshold. The secondary analyses revealed results more in favour of LAPs.
Conclusion: Although the COCP has a slightly higher probability of being cost-effective at £20 000/QALY threshold, there remains considerable uncertainty, with only marginal differences in outcomes between the two treatments. The lower rates of further surgery and second-line medical treatment for women allocated to LAPs may make this option preferable for some women.
Trial registration number: ISRCTN 97865475.
Keywords: GYNAECOLOGY; HEALTH ECONOMICS; Healthcare Costs.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare support from the National Institute for Health and Care Research for the submitted work. SB declares receiving fees from Merck and Ferring. All other authors declare no financial relationships with any organisations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
