High recent PrEP adherence with point-of-care urine tenofovir testing and adherence counselling among young African women: results from the INSIGHT cohort
- PMID: 39653676
- PMCID: PMC11628190
- DOI: 10.1002/jia2.26389
High recent PrEP adherence with point-of-care urine tenofovir testing and adherence counselling among young African women: results from the INSIGHT cohort
Abstract
Introduction: Adolescent girls and young women (AGYW) account for two-thirds of new HIV infections in Africa. African AGYW have had high uptake of oral HIV pre-exposure prophylaxis (PrEP) but low adherence, which might be improved by point-of-care adherence monitoring with tailored counselling.
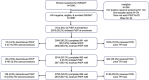
Methods: From August 2022 to July 2023, we conducted a PrEP demonstration project with sexually active AGYW ages 16-30 years from 20 sites in South Africa, Eswatini, Kenya, Malawi, Uganda and Zambia. Participants were offered oral tenofovir-based PrEP at enrolment and followed up at 1, 3 and 6 months. PrEP adherence was assessed by a point-of-care qualitative lateral flow urine tenofovir (TFV) assay indicating PrEP use in the prior 4 days, which accompanied real-time adherence counselling that incorporated urine TFV results when testing was available (70.8% of month 1, 35.3% of month 3 and 83.9% of month 6 visits). We estimated overall adherence, correcting for missing test results, and analysed the association of having received urine TFV results at month 1 or 3 with subsequent urine TFV test positivity, using modified Poisson regression.
Results: Of the 3087 AGYW enrolled, the median age was 24 years (interquartile range 21-27), 75.7% were from South Africa, 2878 (93.2%) initiated PrEP at enrolment and 107 (3.5%) after enrolment. Visit retention was 92.0-96.2% for months 1, 3 and 6, and 2518 (90.1%) exited the study with a PrEP refill. Adherence, based on the point-of-care urine tenofovir test positivity rate, was estimated as 72%, 71% and 65% at months 1, 3 and 6, respectively. Women who received one prior urine TFV test had a 42% higher likelihood of a subsequent positive urine TFV test (adjusted odds ratio, OR = 1.42, 95% confidence interval, CI 1.27-1.60), and those having received two prior tests had a 67% higher likelihood (adjusted OR = 1.67; 95% CI 1.41-1.98). Observed HIV incidence was 1.38/100 person-years (95% CI 0.97-2.08).
Conclusions: Oral PrEP uptake, recent adherence and persistence were high in a multisite cohort of young African women over 6 months of follow-up. The use of a novel point-of-care tenofovir assay with tailored real-time adherence counselling was associated with increased adherence to PrEP at subsequent visits, warranting further study.
Clinical trials registration: clinicaltrials.gov NCT05746065.
Keywords: Africa; PrEP; adherence; point‐of‐care; tenofovir; women.
© 2024 The Author(s). Journal of the International AIDS Society published by John Wiley & Sons Ltd on behalf of International AIDS Society.
Conflict of interest statement
BGM, MK, ZZ, EB, RP, CL, NM, PS, MS, LN, RC, MK, HN‐B, PK, KG, PM, AvH, SB, MJ, PdP, AW, RPHP, SD‐M, SP, RJ, JC and SM have nothing to disclose. SD‐M, RH and MG have received NIH research grants. DD and JV have served as a DSMB member on NIH‐funded grants and received NIH research grants. CC has received honoraria from Gilead and Merck as a scientific advisor, unrelated to this research, and research grants from NIH and BMGF.
Figures
References
-
- UNAIDS . The path that ends AIDS: UNAIDS Global AIDS Update. 2023. Accessed November 12, 2024. Available from: https://www.unaids.org/en/resources/documents/2023/global‐aids‐update‐2023
-
- Celum C, Hosek S, Tsholwana M, Kassim S, Mukaka S, Dye BJ, et al. PrEP uptake, persistence, adherence, and effect of retrospective drug level feedback on PrEP adherence among young women in southern Africa: results from HPTN 082, a randomized controlled trial. PLoS Med. 2021;18(6):e1003670. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

