The antitumor activity of TGFβ-specific T cells is dependent on IL-6 signaling
- PMID: 39653766
- PMCID: PMC11685413
- DOI: 10.1038/s41423-024-01238-7
The antitumor activity of TGFβ-specific T cells is dependent on IL-6 signaling
Abstract
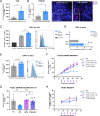
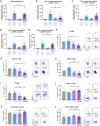
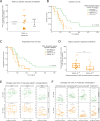
Although interleukin (IL)-6 is considered immunosuppressive and tumor-promoting, emerging evidence suggests that it may support antitumor immunity. While combining immune checkpoint inhibitors (ICIs) and radiotherapy in patients with pancreatic cancer (PC) has yielded promising clinical results, the addition of an anti-IL-6 receptor (IL-6R) antibody has failed to elicit clinical benefits. Notably, a robust TGFβ-specific immune response at baseline in PC patients treated solely with ICIs and radiotherapy correlated with improved survival. Recent preclinical studies demonstrated the efficacy of a TGFβ-based immune modulatory vaccine in controlling PC tumor growth, underscoring the important role of TGFβ-specific immunity in PC. Here, we explored the importance of IL-6 for TGFβ-specific immunity in PC. In a murine model of PC, coadministration of the TGFβ-based immune modulatory vaccine with an anti-IL-6R antibody rendered the vaccine ineffective. IL-6R blockade hampered the development of vaccine-induced T-cells and tumoral T-cell infiltration. Furthermore, it impaired the myeloid population, resulting in increased tumor-associated macrophage infiltration and an enhanced immunosuppressive phenotype. In PC patients, in contrast to those receiving only ICIs and radiotherapy, robust TGFβ-specific T-cell responses at baseline did not correlate with improved survival in patients receiving ICIs, radiotherapy and IL-6R blockade. Peripheral blood immunophenotyping revealed that IL-6R blockade altered the T-cell and monocytic compartments, which was consistent with the findings in the murine model. Our data suggest that the antitumor efficacy of TGFβ-specific T cells in PC depends on the presence of IL-6 within the tumor. Consequently, caution should be exercised when employing IL-6R blockade in patients receiving cancer immunotherapy.
Keywords: IL-6; TGFβ; immunosuppression; tumor microenvironment; vaccines.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: IL is an employee at IO Biotech. IMS has received research funding from Evaxion Biotech, Adaptimmune, IO Biotech, Lytix Biopharma, TILT Biotherapeutics, Enara Bio, and Asgard Biotech. IMS has received consulting fees for MSD, IO Biotech, Novartis, Pierre Fabre, and TILT Biotherapeutics. The IMS has received travel support from the MSD. IMS has received payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing, or educational events from MSD, Novartis, Sanofi Aventis, Pierre Fabre, BMS, Novo Nordisk, and Takeda. MD is an advisor of Achilles Therapeutics and has received access to proprietary data from Bristol Myers Squibb and Genentech. IMC has received research funding from Roche, Bristol Myers Squibb, Celgene, Genis, and Varian Medical Systems, AstraZeneca, and Genmab. Travel and accommodation expenses for IMC have been covered by Roche, Bristol Myers Squibb, Celgene, Bayer, and AstraZeneca. The IMC has an advisory role at Amgen, AstraZeneca, and ANOCCA. MHA has developed an invention based on the use of transforming growth factor-β–derived peptides for vaccinations. A patent application directed to the invention is owned by the company IO Biotech ApS and lists the MHA as the sole inventor. MHA is an advisor and shareholder at IO Biotech. The additional authors declare that they have no competing interests.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

