The use of automated insulin delivery around physical activity and exercise in type 1 diabetes: a position statement of the European Association for the Study of Diabetes (EASD) and the International Society for Pediatric and Adolescent Diabetes (ISPAD)
- PMID: 39653802
- PMCID: PMC11732933
- DOI: 10.1007/s00125-024-06308-z
The use of automated insulin delivery around physical activity and exercise in type 1 diabetes: a position statement of the European Association for the Study of Diabetes (EASD) and the International Society for Pediatric and Adolescent Diabetes (ISPAD)
Abstract
Regular physical activity and exercise (PA) are cornerstones of diabetes care for individuals with type 1 diabetes. In recent years, the availability of automated insulin delivery (AID) systems has improved the ability of people with type 1 diabetes to achieve the recommended glucose target ranges. PA provide additional health benefits but can cause glucose fluctuations, which challenges current AID systems. While an increasing number of clinical trials and reviews are being published on different AID systems and PA, it seems prudent at this time to collate this information and develop a position statement on the topic. This joint European Association for the Study of Diabetes (EASD)/International Society for Pediatric and Adolescent Diabetes (ISPAD) position statement reviews current evidence on AID systems and provides detailed clinical practice points for managing PA in children, adolescents and adults with type 1 diabetes using AID technology. It discusses each commercially available AID system individually and provides guidance on their use in PA. Additionally, it addresses different glucose responses to PA and provides stratified therapy options to maintain glucose levels within the target ranges for these age groups.
Keywords: Automated insulin delivery; CGM; Exercise; Glucose; Insulin pump; Physical activity; Position statement; Type 1 diabetes.
© 2024. The Author(s).
Conflict of interest statement
Acknowledgements: We would like to thank the nine reviewers (three experts in the field of PA, AID and type 1 diabetes; three people with type 1 diabetes using AID systems; and three parents of children/adolescents with type 1 diabetes using AID systems) for critical assessment of this manuscript prior to submission to the EASD CCA. We also want to thank K. Moser for support with the production of the figures and tables. We extend our gratitude to all individuals living with type 1 diabetes who have generously contributed their time and participated in research trials. Finally, we would like to acknowledge the following individuals who have contributed to clinical trials, data collection and advancing the field of exercise and type 1 diabetes research: D. Morrison (University of Melbourne, Australia), B. Paldus (University of Melbourne, Australia), O. McCarthy, (Steno Diabetes Center Copenhagen, Denmark), G. Ash (Yale University, USA), L. Nally (Yale University, USA), M. Clements (Children’s Mercy, Kansas City, USA), R. Gal (Jaeb Center for Health Research, USA), S. Patton (Nemours Children's Health, USA), N. Bratina (University of Ljubljana, Slovenia), F. Annan (University College London Hospitals NHS Foundation Trust, UK), C. Smart (John Hunter Children’s Hospital, Australia), E. Heyman (University of Lille, France) and S. Tagougui (University of Lille, France). Data availability: All data used within this position statement are included in the manuscript. Funding: Open Access funding enabled and organized by Projekt DEAL. OM has received research funding from Sêr Cymru II COFUND Fellowship/European Union, Novo Nordisk, Abbott Diabetes Care, Sanofi, Dexcom, Team Novo Nordisk, SAIL, Brauerei Maisel, Medtronic, European Foundation for the Study of Diabetes (EFSD)/EASD, Falke, BISp., Ypsomed, DiabetesDE and Perfood. DPZ has received an ISPAD-JDRF Research Fellowship and research support from Insulet and the Leona M. and Harry B. Helmsley Charitable Trust. PA has received research grants from Medtronic and Novo Nordisk. KD has received an ISPAD-JDRF Research Fellowship. TB’s institution has received research grant support from Abbott, Medtronic, Novo Nordisk, Sanofi, Novartis, Sandoz, Zealand Pharma, the Slovenian Research Agency, the National Institutes of Health (NIH) and the European Union. Yale School of Medicine has received research support for JLS from Abbott Diabetes, the Jaeb Center for Health Research, JDRF, Insulet, Medtronic, NIH and Provention Bio. JEY has received in-kind research support from Dexcom and LifeScan Canada and support from Medtronic, Dexcom and Abbott. KN’s institution has received research funding from Zealand Pharma, Novo Nordisk, Medtronic and Dexcom. HS has received research support from Boehringer Ingelheim, Eli Lilly, Novo Nordisk, the European Union, the Austrian Research Promotion Agency (FFG) and the Austrian Science Fund (KLP3413324). BAB has received research support from the NIDDK and Breakthrough T1D. KU Leuven has received research support for CM from Medtronic, Novo Nordisk, Sanofi and ActoBio Therapeutics. MCR has received research grants and/or contract research support from the Jaeb Center for Health Research, Sanofi, Novo Nordisk, Eli Lilly, Dexcom, Insulet and Zucara Therapeutics. GPF has received research funding from NIH, JDRF/BT1D, NSF, Medtronic, Dexcom, Abbott, Tandem, Insulet, Beta Bionics and Lilly. EAD’s institution has received research support from Medtronic, Dexcom and Tandem. NSO has received research support from Dexcom, Medtronic, Roche Diabetes, Diabetes UK, Imperial College NIHR BRC, the Leona M. and Harry B. Helmsley Charitable Trust and UKRI EME. For PG, KU Leuven has received research grants from Dexcom (financial and non-financial support), Medtronic, Novo Nordisk, Roche, Sanofi and Tandem. PG is the recipient of a senior clinical research fellowship from FWO, the Flemish Research Council (1861924N). RMB has received research funding or product supply from Medtronic, Novo Nordisk, Sêr Cymru II COFUND European Union, Abbott Diabetes Care, Sanofi, Dexcom, Team Novo Nordisk, Supersapiens and Beneo. RH reports receiving speaker honoraria from Eli Lilly, Dexcom and Novo Nordisk and license and/or consultancy fees from B. Braun and Abbott Diabetes Care, patents related to closed-loop, and being director at CamDiab. TD serves as Chief Medical Officer of Breakthrough T1D (formerly JDRF). None of the funders played a role in the development of this position paper. Authors’ relationships and activities: OM has received lecture fees from Medtronic, Eli Lilly, Novo Nordisk, Sanofi, TAD Pharma, Theras, Diatec, Ypsomed, Dexcom, AstraZeneca, Insulet and Perfood; and is on the advisory board for Sanofi, TAD Pharma, Glaice, Perfood, Medtronic and Dexcom. DPZ has received honoraria for speaking engagements from Ascensia Diabetes Care, Insulet Canada, Dexcom Canada and Medtronic; is on an advisory board for Dexcom and the Diabetes Research Hub; PA has taken part in advisory boards of Eli Lilly, Insulet, Medtronic, Novo Nordisk, Roche and Sanofi. KD has received honoraria for speaking engagements from Abbott, Eli Lilly, Medtronic, Novo Nordisk and Pfizer; is on an advisory board for Medtronic and Novo Nordisk. SEH has received speaker’s honoraria from Eli Lilly, Sanofi, Medtronic, Insulet, Dexcom and Ypsomed. JP has received speaker’s honoraria from Dexcom and Abbott and sits on the advisory board for Roche. TB has served on advisory panels of Novo Nordisk, Sanofi, Eli Lilly, Boehringer, Medtronic, Abbott and Indigo Diabetes and has received honoraria for participating on the speakers bureaus of Eli Lilly, Novo Nordisk, Medtronic, Abbott, Sanofi, Dexcom, Aventis, AstraZeneca and Roche. JLS reports serving, or having served, on advisory panels for Cecelia Health, Insulet, Medtronic Diabetes, StartUp Health Diabetes Moonshot and Vertex and having served as a consultant to Abbott Diabetes, Insulet, Medtronic Diabetes, Vertex and Zealand. RR-L has received consulting/advisory panel honoraria from Abbott, AstraZeneca, Bayer, Boehringer Ingelheim, Dexcom, Eli Lilly, HLS Therapeutics, INESSS, Insulet, Janssen, Medtronic, Merck, Novo Nordisk, Pfizer and Sanofi-Aventis; honoraria for conferences from Abbott, AstraZeneca, Boehringer Ingelheim, CPD Network, Dexcom, CMS Canadian Medical & Surgical Knowledge Translation Research Group, Eli Lilly, Janssen, Medtronic, Merck, Novo Nordisk, Sanofi-Aventis, Tandem and Vertex Pharmaceutical; consumable gifts (in kind) from Eli Lilly and Medtronic; and purchase fees from Eli Lilly in the field of automated insulin delivery. JEY has received speaker’s fees from Dexcom and Abbott. JKM is a member of the advisory boards of Abbott Diabetes Care, Becton-Dickinson/Embecta, Biomea, Eli Lilly, Medtronic, Novo Nordisk, Pharmasens, Roche Diabetes Care, Sanofi and Viatris; has received speaker honoraria from Abbott Diabetes Care, A. Menarini Diagnostics, Becton-Dickinson/Embecta, Boehringer Ingelheim, Eli Lilly, MedTrust, Novo Nordisk, Roche Diabetes Care, Sanofi, Servier and Ypsomed; and is a shareholder of decide Clinical Software and elyte Diagnostics. MT has received honoraria for speaking engagements from Ely Lilly, Medtronic and Ypsomed and is on advisory boards for Abbott Diabetes Care and Sanofi. KN owns shares in Novo Nordisk and has been a paid consultant for Novo Nordisk and Medtronic and has received speaker and advisory board honorarium for her institution from Abbott, Medtronic, Novo Nordisk, Insulet and Convatec. HS has received speaker’s honoraria or is on the advisory board for Amgen, Amarin, Boehringer Ingelheim, Cancom, Eli Lilly, Daiichi Sankyo and Novo Nordisk. MCR serves on advisory panels for Zealand Pharma, Zucara Therapeutics and Indigo Diabetes; acts as a consultant for the Jaeb Center for Health Research; has given lectures sponsored by Dexcom, Novo Nordisk and Sanofi; and is a shareholder, or holds stocks in, Supersapiens and Zucara Therapeutics. BAB has received consulting fees from Ypsomed, Arecor and Medtronic. CM serves or has served on the advisory panel for Novo Nordisk, Sanofi, Eli Lilly, Novartis, Dexcom, Boehringer Ingelheim, Bayer, Roche, Medtronic, Insulet, Biomea Fusion, SAB Bio and Vertex. Financial compensation for these activities has been received by KU Leuven. CM serves or has served on the speakers bureau for Novo Nordisk, Sanofi, Eli Lilly, Medtronic and Boehringer Ingelheim. Financial compensation for these activities has been received by KU Leuven. CM is president of EASD. All external support provided to EASD can be found at http://www.easd.org/. DNO has received honoraria from Medtronic, Insulet, Abbott Diabetes Care, Novo Nordisk and Sanofi, and research support from Medtronic, Insulet, Dexcom, Roche, GlySens, BioCapillary and Endogenex, and is on advisory boards for Medtronic, Insulet, Abbott Diabetes Care, Ypsomed, Novo Nordisk and Sanofi. GPF has served as a speaker/consultant/ad board member for Medtronic, Dexcom, Abbott, Tandem, Insulet, Beta Bionics, Sequel and Lilly. EAD’s institution has received speaker honorariums from Eli Lilly and EAD has sat on an advisory panel for Tandem. NSO has participated in advisory groups for Dexcom, Medtronic and Roche Diabetes and received fees for speaking from Astra Zeneca, Sanofi, Dexcom, Tandem, Medtronic and Roche Diabetes. PG has served on the advisory board for Insulet and Ypsomed. PG reports consulting fees from Abbott, Bayer and Medtronic, and honoraria for speaking from Abbott, Bayer, Dexcom, Insulet, Medtronic, Novo Nordisk, Vitalaire and Ypsomed (financial compensation received by KU Leuven). PG has received support for attending (virtual) conferences/meetings from Medtronic, Novo Nordisk, Roche and Sanofi (financial compensation received by KU Leuven). RMB has received lecture fees from Abbott Diabetes Care, Novo Nordisk, Medtronic, Eli Lilly and Sanofi. TD has received speaker, advisory panel or research support from Abbott, Dexcom, Eli Lilly, Insulet, Medtronic, Novo Nordisk, Roche, Sanofi, Ypsomed and Vitalaire. He is a shareholder of DreaMed Ltd. The authors declare that there are no other relationships or activities that might bias, or be perceived to bias, their work. Contribution statement: All authors of this joint EASD/ISPAD position statement substantially contributed to conception and design, acquisition of data or analysis and interpretation of data, drafted the article or revised it critically for important intellectual content and approved the final version to be published.
Figures



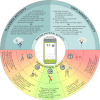

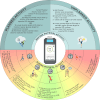

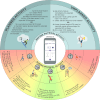

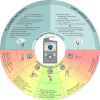

References
-
- Moser O, Riddell MC, Eckstein ML et al (2020) Glucose management for exercise using continuous glucose monitoring (CGM) and intermittently scanned CGM (isCGM) systems in type 1 diabetes: position statement of the European Association for the Study of Diabetes (EASD) and of the International Society f. Diabetologia 63(12):2501–2520. 10.1007/s00125-020-05263-9 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

