Thermally induced neuronal plasticity in the hypothalamus mediates heat tolerance
- PMID: 39653806
- PMCID: PMC11802458
- DOI: 10.1038/s41593-024-01830-0
Thermally induced neuronal plasticity in the hypothalamus mediates heat tolerance
Abstract
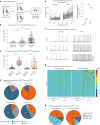
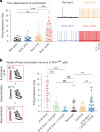
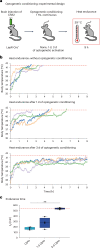
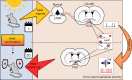
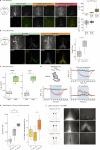
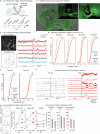
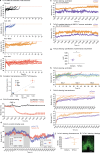
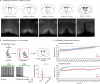
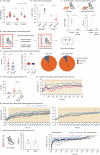
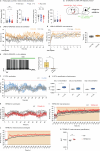
Heat acclimation is an adaptive process that improves physiological performance and supports survival in the face of increasing environmental temperatures, but the underlying mechanisms are not well understood. Here we identified a discrete group of neurons in the mouse hypothalamic preoptic area (POA) that rheostatically increase their activity over the course of heat acclimation, a property required for mice to become heat tolerant. In non-acclimated mice, peripheral thermoafferent pathways via the parabrachial nucleus activate POA neurons and mediate acute heat-defense mechanisms. However, long-term heat exposure promotes the POA neurons to gain intrinsically warm-sensitive activity, independent of thermoafferent parabrachial input. This newly gained cell-autonomous warm sensitivity is required to recruit peripheral heat tolerance mechanisms in acclimated animals. This pacemaker-like, warm-sensitive activity is driven by a combination of increased sodium leak current and enhanced utilization of the NaV1.3 ion channel. We propose that this salient neuronal plasticity mechanism adaptively drives acclimation to promote heat tolerance.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: S.L. is a Novo Nordisk employee and minor shareholder as part of an employee-offering program. The other authors declare no competing interests.
Figures








References
-
- Armstrong, L. E. & Stoppani, J. Central nervous system control of heat acclimation adaptations: an emerging paradigm. Rev. Neurosci.13, 271–285 (2002). - PubMed
-
- Barry, H. et al. Improved neural control of body temperature following heat acclimation in humans. J. Physiol.598, 1223–1234 (2020). - PubMed
-
- Horowitz, M. Heat acclimation: phenotypic plasticity and cues to the underlying molecular mechanisms. J. Therm. Biol.26, 357–363 (2001).
-
- Pierau, F. K., Sann, H., Yakimova, K. S. & Haug, P. Plasticity of hypothalamic temperature-sensitive neurons. Prog. Brain Res.115, 63–84 (1998). - PubMed
MeSH terms
Grants and funding
- ERC-CoG-772395/EC | EU Framework Programme for Research and Innovation H2020 | H2020 Priority Excellent Science | H2020 European Research Council (H2020 Excellent Science - European Research Council)
- SFB/TRR 152/Deutsche Forschungsgemeinschaft (German Research Foundation)
- SFB1158/Deutsche Forschungsgemeinschaft (German Research Foundation)
- Long-term fellowship/European Molecular Biology Organization (EMBO)
- LT000762/2019-L/Human Frontier Science Program (HFSP)
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

