Impact of levetiracetam use in glioblastoma: an individual patient-level meta-analysis assessing overall survival
- PMID: 39653818
- PMCID: PMC11628436
- DOI: 10.1007/s10143-024-03137-x
Impact of levetiracetam use in glioblastoma: an individual patient-level meta-analysis assessing overall survival
Abstract
Background: Levetiracetam (Lev), an antiepileptic drug (AED), enhances alkylating chemotherapy sensitivity in glioblastoma (GB) by inhibiting MGMT expression. This meta-analysis evaluates Lev's impact on GB treatment by analyzing overall survival of individual patient data (IPD) from published studies.
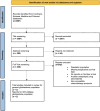
Methods: IPD was reconstructed using the R package IPDfromKM. Pooled IPD Kaplan-Meier charts of survival stratified by Lev therapy were created using the R package Survminer. One- and two-stage meta-analyses of Lev treatment regarding survival was performed.
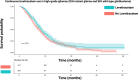
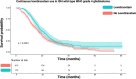
Results: Three articles covering 825 patients were included out of 3567 screened records. Lev usage prevalence was 0.36. IPD from 590 IDH wild-type glioblastomas, with a median follow-up of 16.1 months, were utilized. Pooled data revealed median survival times of 19.2 months (95%CI: 16.4-22.0) for Lev users versus 16.5 months (95%CI: 15.2-17.8) for partial/no use (p = 0.006). One-stage meta-analysis indicated a significant association between Lev use and survival in IDH wild-type GB (HR: 1.33, 95%CI: 1.08-1.64, p = 0.007). Two-stage meta-analysis confirmed these results.
Conclusions: This meta-analysis highlights that Lev use may prolong survival in IDH wild-type GB patients. Further randomized trials are needed to confirm these findings and identify subgroups benefiting most from Lev treatment.
Keywords: Anti-epileptic drug; Glioblastoma; Individual Patient Data; Levetiracetam; Overall survival.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval/study registration: This study is a meta-analysis and ethical approval was waived due to secondary data analysis. The study protocol was prospectively registered in the 'International Prospective Register of Systematic Reviews' (PROSPERO, Registration ID: CRD42024507697). Competing interests: The authors declare no competing interests.
Figures
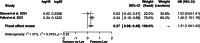
References
-
- Avila EK, Tobochnik S, Inati SK, Koekkoek JAF, McKhann GM, Riviello JJ, Rudà R, Schiff D, Tatum WO, Templer JW, Weller M, Wen PY (2024) Brain tumor-related epilepsy management: a Society for Neuro-oncology (SNO) consensus review on current management. Neuro Oncol 26(1):7–24. 10.1093/neuonc/noad154 - DOI - PMC - PubMed
-
- Pallud J, Le Van Quyen M, Bielle F, Pellegrino C, Varlet P, Cresto N, Baulac M, Duyckaerts C, Kourdougli N, Chazal G, Devaux B, Rivera C, Miles R, Capelle L, Huberfeld G (2014) Cortical GABAergic excitation contributes to epileptic activities around human glioma. Sci Transl Med. 6(244):244ra89. 10.1126/scitranslmed.3008065 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

